Abstract
BACKGROUND AND PURPOSE: Recent data suggest that intra-arterial thrombolytics may be a safe rescue therapy for patients with acute ischemic stroke after unsuccessful mechanical thrombectomy; however, safety and efficacy remain unclear. Here, we evaluate the use of intra-arterial rtPA as a rescue therapy in the Systematic Evaluation of Patients Treated with Neurothrombectomy Devices for Acute Ischemic Stroke (STRATIS) registry.
MATERIALS AND METHODS: STRATIS was a prospective, multicenter, observational study of patients with acute ischemic stroke with large-vessel occlusions treated with the Solitaire stent retriever as the first-line therapy within 8 hours from symptom onset. Clinical and angiographic outcomes were compared in patients having rescue therapy treated with and without intra-arterial rtPA. Unsuccessful mechanical thrombectomy was defined as any use of rescue therapy.
RESULTS: A total of 212/984 (21.5%) patients received rescue therapy, of which 83 (39.2%) and 129 (60.8%) were in the no intra-arterial rtPA and intra-arterial rtPA groups, respectively. Most occlusions were M1, with 43.4% in the no intra-arterial rtPA group and 55.0% in the intra-arterial rtPA group (P = .12). The median intra-arterial rtPA dose was 4 mg (interquartile range = 2–12 mg). A trend toward higher rates of substantial reperfusion (modified TICI ≥ 2b) (84.7% versus 73.0%, P = .08), good functional outcome (59.2% versus 46.6%, P = .10), and lower rates of mortality (13.3% versus 23.3%, P = .08) was seen in the intra-arterial rtPA cohort. Rates of symptomatic intracranial hemorrhage did not differ (0% versus 1.6%, P = .54).
CONCLUSIONS: Use of intra-arterial rtPA as a rescue therapy after unsuccessful mechanical thrombectomy was not associated with an increased risk of symptomatic intracranial hemorrhage or mortality. Randomized clinical trials are needed to understand the safety and efficacy of intra-arterial thrombolysis as a rescue therapy after mechanical thrombectomy.
ABBREVIATIONS:
- IA
- intra-arterial
- MT
- mechanical thrombectomy
- RT
- rescue therapy
- sICH
- symptomatic intracranial hemorrhage
- STRATIS
- Systematic Evaluation of Patients Treated with Neurothrombectomy Devices for Acute Ischemic Stroke
Mechanical thrombectomy (MT) is a powerful therapy for patients with acute ischemic stroke with large-vessel occlusions. However, despite its proved success,1⇓⇓⇓-5 most patients do not achieve complete reperfusion6⇓⇓-9 and only about half of all patients treated with MT achieve a good clinical outcome at 3 months.6 Because patients with complete reperfusion are 2 times more likely to have favorable outcomes than those with near-complete reperfusion,10 exploration of adjunctive or rescue therapies (RTs) to augment MT complete reperfusion is warranted.
The role of intra-arterial (IA) thrombolysis has evolved from a primary therapy11⇓⇓⇓⇓⇓-17 to an adjunctive or RT to MT. Recently, a US survey indicated that 60.6% of neurointerventionalists use IA lytics in their practice, with the most common approach as an RT after MT.18 Previous studies on the use of IA rtPA in the context of MT either as an RT or adjunctive therapy have yielded promising data, but these studies are limited by their small sample sizes and retrospective design.19⇓-21 Here, in this subanalysis, we retrospectively evaluate the use of IA rtPA as an RT after unsuccessful MT in the multicenter, prospective, Systematic Evaluation of Patients Treated with Neurothrombectomy Devices for Acute Ischemic Stroke (STRATIS) registry (https://www.clinicaltrials.gov/ct2/show/NCT02239640?term=STRATIS&draw=2&rank=7).
MATERIALS AND METHODS
Study Population
STRATIS Registry.
The STRATIS registry was a prospective, multicenter, nonrandomized, observational study that evaluated the use of the Solitaire revascularization device (Medtronic) and MindFrame Capture low-profile revascularization device (Medtronic) in 1000 patients with anterior circulation large-vessel occlusions between August 2014 and June 2016 at 55 US centers (https://www.clinicaltrials.gov; unique identifier: NCT02239640). Ethics approval was obtained by the institutional review board at each center. Before enrollment in the registry, each subject provided written informed consent. The details and results of the STRATIS registry are published elsewhere.7 Briefly, key inclusion criteria were the following: 1) confirmed, symptomatic intracranial large-vessel occlusion with associated symptoms; 2) an NIHSS score of 8 to thirty; 3) use of the Medtronic market-released neurothrombectomy device as the initial device; 4) premorbid mRS of ≤1; and 5) treatment within 8 hours of stroke onset. Procedural information was obtained via core lab analysis of the de-identified complete reports and complete procedural imaging. RT was defined in the STRATIS registry as any mechanical device or thrombolytic used after the primary neurothrombectomy device (Solitaire or MindFrame). MindFrame was recalled on February 26, 2018, due to a risk of the delivery wire breaking or separating during use.
IA Subanalysis
For this subgroup analysis, all patients who underwent RT were included. Within this RT group, patients with and without IA rtPA use were compared. An additional subgroup analysis was also performed in patients with RT with M1 occlusions only. Baseline and procedural characteristics and outcomes were compared between the 2 subgroups. Clinical outcomes at 90 days included an mRS score and mortality. Safety outcomes included the incidence of symptomatic intracranial hemorrhage (sICH).
Statistical Analysis
Standard descriptive statistics, in-cluding mean [SD] and median with interquartile range, were used for continuous variables, and frequency distributions, for categoric variables. For between-group comparisons, χ2 tests and the Fisher exact test were used for categoric variables, while t tests and Wilcoxon rank sum tests were used for continuous variables. All P values were 2-sided, and values ≤.05 were considered significant. Multivariable logistic regression models were fit for the following outcomes: substantial reperfusion, good functional outcomes (mRS 0–2), and mortality at 90 days. Clinically relevant variables and variables with P < .10 were entered into the models. Statistical analyses were conducted using SAS, Version 9.3 (SAS Institute), and R statistical and computing software, Version 3.2 (http://www.r-project.org).
RESULTS
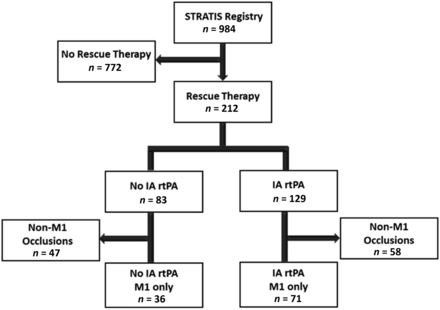
Of the 984 patients in the STRATIS registry, 212 (21.5%) underwent RT and were included in this analysis (Fig 1). From the RT cohort, 129 patients (60.8%) received IA rtPA.
Study flow chart.
Baseline demographics were balanced between the groups, with the exception of hyperlipidemia, which was more prevalent in the no IA rtPA group (56.6% versus 41.1%, P = .03) (Online Supplemental Data). There was no difference in the mean NIHSS scores at presentation (17.6 [SD, 5.7] versus 17.0 [SD, 5.5], P = .43). Most patients in both cohorts received IV rtPA (62.7% versus 70.5%, P = .24) and had an anterior circulation occlusion (95.2% versus 93.8%, P = .09).
The mean time of onset to groin puncture was significantly shorter in the IA rtPA group (227.9 [SD, 91.9] versus 200.2 [SD, 104] minutes, P = .05). Only ∼11% of patients in each group had an onset-to-groin puncture time of >6 hours. The mean number of passes was lower in the IA rtPA group (3.6 [SD, 1.3] versus 2.2 [SD, 1.4], P ≤ .001); however, the number of passes in both groups did not differ before RT (Online Supplemental Data). The median dose of IA rtPA administered was 4 mg (interquartile range = 2–12 mg). There was a trend toward faster procedural times in the IA rtPA group (89.1 [SD, 45.1] versus 78.7 [SD, 43.1] minutes, P = .10). The rate of substantial reperfusion (modified TICI ≥ 2b) was numerically higher in the IA rtPA group (73.0% versus 84.7%, P = .08) (Online Supplemental Data and Fig 2A).
Outcomes in the RT no IA rtPA versus RT IA rtPA groups. A, Revascularization outcomes. B, Ninety-day mRS in the RT no IA rtPA versus RT IA rtPA groups.
There was a trend toward higher rates of good functional outcome (mRS ≤ 2) (46.6% versus 59.2%, P = .10) and lower rates of mortality (23.3% versus 13.3%, P = .08) at 90 days in the IA rtPA cohort (Online Supplemental Data and Fig 2B). No difference was found in the rate of sICH between the groups (0% versus 1.6%, P = .54).
Multivariable logistic regression analysis when adjusting for IA rtPA use, history of hyperlipidemia, number of device passes, time from onset to procedure end, time from onset to arterial puncture, M1 vessel location, and ICA vessel location did not show IA rtPA use as an independent predictor of substantial reperfusion (OR = 1.07; 95% CI, 0.44–2.57; P = .89), good functional outcome (OR = 0.92; 95% CI, 0.46–1.83; P = .80), or mortality (OR = 0.54; 95% CI, 0.22–1.31; P = .17) (Online Supplemental Data).
When further restricting the RT population to M1 occlusions only, there was no difference in the rates of substantial reperfusion (83.3% versus 90.6%, P = .45), good functional outcome (52.9% versus 60.0%, P = .53), mortality (17.6% versus 10.8%, P = .36), and sICH (0% versus 1.4%, P = 1.0) in the IA rtPA and no IA rtPA groups, respectively (Online Supplemental Data and Figs 1 and 2).
DISCUSSION
In this subgroup analysis of the STRATIS registry, use of IA rtPA in patients with RT did not result in an increased risk of sICH. Furthermore, there was a trend toward higher rates of successful reperfusion and good clinical outcome and lower rates of mortality in patients with RT receiving IA rtPA.
Our study adds to a small-but-growing body of literature that suggests that IA thrombolytics may be a safe and effective adjunctive or RT during or after MT.19⇓⇓⇓-23 A recent subanalysis from the multicenter North American Solitaire Acute Stroke (NASA) registry examined the use of IA rtPA after failed MT and reported numerically higher rates of revascularization success (61.2% versus 46.6%, P = .13) and faster recanalization times (100 [SD, 85] versus 164 [SD, 235] minutes, P = .36) in patients treated with IA rtPA compared with those with no IA rtPA use.19 Most important, the authors reported no difference in the rates of sICH (13.9% versus 6.8%, P = .29) and mortality (42.9% versus 44.7%, P = .13) between the groups. Similarly, a single-center retrospective study by Anadani et al20 reported no difference in rates of successful recanalization, hemorrhage, or mortality at 90 days in patients who received IA rtPA as rescue therapy after MT versus MT only. Kaesmacher et al22 examined the use of IA urokinase after failed or incomplete MT in 100 patients with anterior circulation large-vessel occlusion. Use of IA urokinase in this study was not associated with an increased risk of sICH (adjusted OR, 0.81; 95% CI, 0.31–2.13) or mortality (adjusted OR = 0.78; 95% CI, 0.43–1.40) and resulted in improved angiographic reperfusion.
The safety of IA rtPA after IV rtPA administration and MT has not been well-studied. In the present study, most patients receiving IA rtPA RT (62.7%) received IV rtPA. Because the sICH rates were comparable between the cohorts (0% versus 1.6%, P = .54), our data suggest that IA rtPA administration in the context of IV rtPA and MT appears to be safe. Anadani et al20 investigated the safety of IA rtPA use after IV rtPA and MT and found no difference in the rate of hemorrhagic complications between the IV rtPA/IA rtPA and IA rtPA-only groups; however, only 13 patients received IV rtPA/IA rtPA in the study. Although the randomized MT trials, Multicenter Randomized CLinical trial of Endovascular treatment for Acute ischemic stroke in the Netherlands [MR CLEAN] and Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times Trial, allowed the use of IA rtPA, no data have been published on the safety of IA thrombolytic use in these studies.1,2
Only a few studies have reported IA rtPA dosing during or after MT.20,21,23 The median dose of IA rtPA administered as an RT in our study was 4 mg (interquartile range = 2–12 mg), which was similar to the median dose of 5 mg (interquartile range = 4–6 mg) reported by Anadani et al.20 A recent survey by Castonguay et al18 showed that 60.6% of respondents used IA thrombolytics in their practice and that most used an IA rtPA dose of 3–10 mg; however, 84.9% do not have a standardized protocol for administration. Because the administration protocols, dosing, and indications varied widely among studies that investigated IA rtPA in the context of MT,19⇓-21,23 further study is warranted. Currently, the Chemical Optimization of Cerebral Embolectomy (CHOICE) trial is an ongoing multicenter, randomized, placebo-controlled, double-blinded Phase 2b study to assess the effectiveness of IA thrombolysis (rtPA) after incomplete reperfusion (modified TICI 2b or 2c) with MT.24 Patients will be randomized to receive a 20- to 30-minute intra-arterial infusion of rtPA (up to 22.5 mg) or a placebo. Results from the CHOICE trial will help establish guidance on the potential efficacy and safety of IA rtPA as an RT after MT.
Limitations
This substudy has several limitations. Data reported here were limited to variables captured in the STRATIS registry. Thus, reasons for IA rtPA adminstration, adminstration technique, and infusion times were not available, possibly contributing to selection bias and the generalizability of our study results. Reasons for the choice of RT were not recorded in the STRATIS registry; therefore, selection bias needs to be considered when interpreting the results of the study. Onset-to-groin puncture times were significantly faster in the IA rtPA group, possibly impacting the outcomes in this study. The STRATIS registry enrolled patients and treated patients within 8 hours from symptom onset; therefore, the results from this study cannot be extrapolated to later time windows. The number of passes was significantly higher in the RT no IA rtPA group; however, it was adjusted for in multivariate outcome models. Treatment strategies varying in the RT population may limit the generalizability and interpretability of our results. Additionally, the definition of RT was specific to the STRATIS registry and is not reflective of clinical practice.
CONCLUSIONS
To our knowledge, this is the largest multicenter, core lab–adjudicated cohort of patients with RT and IA rtPA after unsuccessful MT with the Solitaire device. Our results demonstrate similar rates of mortality, sICH, and reperfusion and 90-day clinical outcomes compared with patients having RT with no IA rtPA use. Large, prospective, randomized clinical trials are needed to further investigate the safety and efficacy of IA thrombolysis after unsuccessful MT.
Footnotes
This study was sponsored by Medtronic.
Syed F. Zaidi and Alcia C. Castonguay contributed equally to this work.
Disclosures: Osama O. Zaidat—RELATED: Grant: Medtronic*; Consulting Fee or Honorarium: Medtronic. Nils Mueller-Kronast—RELATED: Support for Travel to Meetings for the Study or Other Purposes: Medtronic, Comments: travel expenses for meeting presentation; Fees for Participation in Review Activities such as Data Monitoring Boards, Statistical Analysis, Endpoint Committees, and the Like: Medtronic, Comments: Steering Committee. David S. Liebeskind—RELATED: Fees for Participation in Review Activities such as Data Monitoring Boards, Statistical Analysis, Endpoint Committees, and the Like: Medtronic, Comments: Imaging Core Lab; UNRELATED: Consultancy: Stryker, Comments: Imaging Core Lab. *Money paid to institution.
References
- Received August 25, 2020.
- Accepted after revision October 21, 2020.
- © 2021 by American Journal of Neuroradiology