Abstract
BACKGROUND AND PURPOSE: Although “corpus callosum agenesis” is an umbrella term for multiple entities, prenatal counseling is based reductively on the presence (associated) or absence (isolated) of additional abnormalities. Our aim was to test the applicability of a fetal MR neuroimaging score in a cohort of fetuses with prenatally diagnosed isolated corpus callosum agenesis and associated corpus callosum agenesis and correlate it with neurodevelopmental outcomes.
MATERIALS AND METHODS: We performed a single-center retrospective analysis of a cohort of cases of consecutive corpus callosum agenesis collected between January 2011 and July 2019. Cases were scored by 2 raters, and interater agreement was calculated. Outcome was assessed by standardized testing (Bayley Scales of Infant and Toddler Development, Kaufman Assessment Battery for Children) or a structured telephone interview and correlated with scores using 2-way ANOVA.
RESULTS: We included 137 cases (74 cases of isolated corpus callosum agenesis), imaged at a mean of 27 gestational weeks. Interrater agreement was excellent (0.98). Scores were higher in associated corpus callosum agenesis (P < .0001) without a significant score difference between complete and partial corpus callosum agenesis (P = .38). Outcome was assessed in 42 children with isolated corpus callosum agenesis and 9 with associated corpus callosum agenesis (mean age, 3.1 years). MR imaging scores correctly predicted developmental outcome in 90.7% of patients with isolated corpus callosum agenesis, improving neurodevelopmental risk stratification in corpus callosum agenesis.
CONCLUSIONS: The scoring system is very reproducible and can differentiate isolated corpus callosum agenesis and associated isolated corpus callosum agenesis (significantly higher scores) but not between partial and complete corpus callosum agenesis. Scores correlated with outcome in isolated corpus callosum agenesis, but there were too few associated postnatal cases of isolated corpus callosum agenesis to draw conclusions in this group.
ABBREVIATIONS:
- aCCA
- associated corpus callosum agenesis
- CC
- corpus callosum
- CCA
- corpus callosum agenesis
- DCC
- deleted in colorectal cancer
- iCCA
- isolated corpus callosum agenesis
- MCD
- malformations of cortical development
- TOP
- termination of pregnancy
Corpus callosum (CC) agenesis (CCA) is one of the most common malformations of the CNS.1 Rather than a single entity, CCA is an umbrella term defined by anatomy, independent of etiology or outcome. To further complicate matters, CCA includes several subtypes, including complete (when the entire CC is missing) and partial (when part but not all of the CC is absent), but CCA often also includes several degrees of hypoplasia (CC present but of reduced dimensions) and dysgenesis (CC present yet malformed). Each option may be seen in isolation (no other fetal brain or body malformations) or in the context of a polymalformative or genetic condition.1⇓-3
In terms of outcome risk stratification, patients with associated CCA (aCCA) are at a high risk of neurodevelopmental delay,4 while isolated CCA (iCCA) is associated with development within the normal range in up to 88% of children.5⇓⇓⇓-9 Other features, such as the presence of Probst bundles or sigmoid bundles, have been used inconsistently in an attempt to predict outcome.10,11 In an attempt to improve risk stratification in iCCA, we developed and tested a score based on anatomic features evaluated on fetal brain MR imaging in patients with detailed postnatal neuropsychological outcomes.12
This study aimed to test the validity of an MR imaging score initially developed for isolated congenital CCA in a heterogeneous group of complete and partial CCA and to correlate it with neurodevelopmental outcomes.
MATERIALS AND METHODS
Patients and Setting
A retrospective cohort of consecutive cases of prenatally diagnosed CCA with fetal MR imaging between January 2011 and September 2019 was collected in a single tertiary care center (Medical University of Vienna).
Fetuses were subdivided into 4 groups based on imaging characteristics (determined on MR imaging by consensus of 2 experts): complete isolated, complete associated, partial isolated, and partial associated CCA. CCA was considered isolated when no other brain, spine, or extra-CNS anomalies were detected on ultrasound or MR imaging antenatally and no chromosomal anomalies were identified, in accordance with previous publications.4,6,13⇓⇓-16 In non-isolated CCA, associated anomalies were recorded. Severe global brain structural malformations that course without a CC (eg, holoprosencephaly, anencephaly) and the absence of the CC secondary to destructive lesions (eg, porencephaly) were excluded.17,18
Imaging Analysis
Images were independently scored by 1 neuroradiologist with 7 years’ experience in fetal MR imaging and a pediatrics resident with 1 year’s basic experience in fetal MR imaging, using a previously published anatomic fetal MR imaging score,12 consisting of 7 categories: gyration, opercularization, temporal lobe symmetry or asymmetry, lamination, hippocampal abnormalities, basal ganglia, and ventricular enlargement in a 0- to 2-point score (Table 1 and Online Supplemental Data), with a maximum attainable score of 11 points. Interrater agreement was calculated.
Summarized outcome by subgroup of corpus callosum agenesis
MR imaging examinations included a detailed evaluation of the fetal CNS and body, following published guidelines.19 For the fetal brain assessment, we obtained T2-weighted single-shot FSE imaging in 3 orthogonal planes (section thickness = 2–4 mm, section gap = 0–0.4 mm, FOV = 230–260 mm, matrix = 256). Body MR imaging evaluation comprised T2-weighted steady-state free precession sequences in 3 orthogonal planes, and T2-weighted spin-echo FSE, T1WI, EPI, and DWI in at least 1 plane. Further sequences were acquired depending on examination findings and fetal and maternal cooperation. No maternal or fetal sedation was administered before the examination.
Neurodevelopmental Assessment
Patients who are followed in our center were evaluated by a neuropediatrician (R.S.) and a psychologist (S.G.) as previously described in detail in a previous publication.12 The Bayley Scales of Infant and Toddler Development, Third Edition, was used for neurodevelopmental assessment of motor control, cognitive functioning, and language skills (1–42.5 months of age). Normal development was defined as a development quotient score of ≥85, and moderate-to-severe developmental delay was defined as a development quotient score of <70. Children older than 42.5 months were tested for cognitive and language skills using the Kaufman Assessment Battery for Children, Second Edition, complemented by the Peabody Developmental Motor Scales, Second Edition or the Bruininks-Oseretsky Test of Motor Proficiency, Second Edition to evaluate motor skills. Normal development was defined as a Global Scale Index of ≥85.
For children not followed up in our center, a structured telephone interview with 1 or both parents or legal guardians was conducted by a trained psychologist (S.G.). Besides assessment of developmental milestones, the need for further support, type of therapy, and type of kindergarten/schooling were also collected.
Statistical Analysis
Metric data are described using mean [SD] and range if normally distributed or median and maximum and minimum values for skewed metric or ordinal data. Additionally, 95% confidence intervals were calculated. Categoric data are presented as absolute frequencies and percentages. One-way ANOVAs and Bonferroni-corrected post hoc tests were used for differences in age among the 4 groups. Two-way ANOVAs were post hoc tests used to compare the groups regarding MR imaging and neurodevelopmental outcome scores to be able to model a moderation effect additionally. The Spearman rank correlation coefficient (ρ) was calculated to describe the correlation between MR imaging and neurodevelopmental variables. Image ratings were summed, and the resulting score was correlated with the neurodevelopmental outcome scores. Crosstabs and χ2 tests were used to compare groups regarding nominal data.
A P value ≤ 5% (P = .05) indicated significant results. To avoid an increasing error of the second type, we did not perform multiplicity corrections. All analyses were performed using SPSS Statistics for Window, Version 26.0 (IBM).
RESULTS
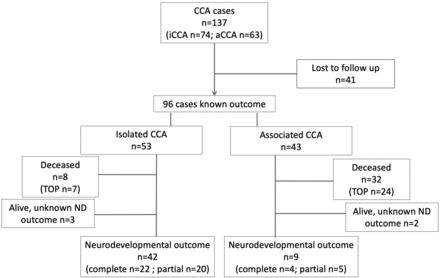
A total of 137 cases of CCA were imaged in our center during the study period (Fig 1), with a mean gestational age of 27 gestational weeks (range, 19 + 4 to 37 + 1 gestational weeks). Of these, 74 fetuses (complete: n = 45; partial n = 29) presented with iCCA, while 63 fetuses (complete: n = 32; partial n = 31) had associated conditions. The most common associated anomalies involved the CNS (n = 58/64), mostly involving the supratentorial brain and specifically malformations of cortical development (MCD) (MCD: n = 34/64; only MCD: n = 16; MCD with other associated anomalies: n = 18), followed by posterior fossa anomalies (n = 23/64). There were extra-CNS anomalies in 31 fetuses, and these were the only findings besides CCA in 6 cases (congenital diaphragmatic hernia, n = 1; heart malformation, n = 2; limb anomalies alone, n = 1; and in association with head and neck malformations, n = 2).
Flowchart detailing patient inclusion and exclusion criteria. ND indicates neurodevelopmental.
Interrater agreement was excellent (0.98).
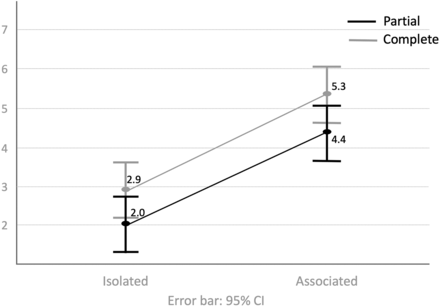
By means of the MR imaging score, there was a statistically significant difference between associated and complete CCA (P < .0001), but not between partial and complete CCA within each group (mean iCCA: partial = 2.69 [SD, 0.31], 95% CI, 2.07–3.31; complete = 2.04 [SD, 0.38], 95% CI, 1.28–2.80; aCCA: partial = 4.92 [SD, 0.37], 95% CI, 4.19–5.65; complete = 4.96 [SD, 0.37], 95% CI, 4.23–5.69; P = .38) (Fig 2).
Comparison of mean MR imaging scores and 95% confidence intervals among the 4 CCA groups: isolated (complete and partial) and associated (complete and partial).
Forty-one cases were lost to follow-up, leaving 96 pregnancies with known outcomes (Fig 1). Parents opted for termination of pregnancy (TOP) more often in the aCCA subgroup (aCCA, n = 24/43, [55.8%]; iCCA, n = 7/53, [13.2%]; P < .001). There were also more cases of natural deaths in this group (aCCA n = 8), 4 cases in each complete and partial aCCA group; 3 children were stillborn (gestational age unknown); and 5 died after birth. In iCCA, all TOPs were in the complete iCCA group.
In 3 cases of iCCA and 2 of aCCA, children were alive, but no detailed follow-up was available. In the 51 remaining cases, the mean age at the time of evaluation or interview was 3.1 [SD, 2.08] years, and there was no significant difference in the mean age of the 4 subgroups (iCCA: complete = 3.07 [SD, 2.36] years; partial = 2.76 [SD, 1.36] years; aCCA: complete = 4.38 [SD, 2.69] years; partial = 3.96 [SD, 2.46] years; P = .42).
Outcome was assessed in the remaining 42 cases of iCCA and 9 of aCCA. The neurodevelopmental outcome overall and divided into domains (speech, cognition, and motor) and the need for special schooling and therapy are summarized in the Table and Online Supplemental Data. In the iCCA group, 31/42 patients (73.8%) had normal development, with all school-age children attending regular school in the appropriate year, without need for special education. By means of the established cutoff of ≤3 for good neurodevelopmental outcome and ≥4 for a high risk of neurodevelopmental delay, MR imaging was correct in 90.7% of cases. In the normally developing group, there was 1 fetus with a high MR imaging score of 4. In the remaining 96.8% (30/31) of cases, the score predicted the favorable outcome correctly. In the developmental delay group, the MR imaging score was incorrect in 4/43 cases (9.3%). There were too few cases in the associated group to evaluate the correlation of scoring and outcome (Fig 3). Due to the small sample size for iCCA, it was not possible to adequately compare the outcomes in the associated and isolated groups further.
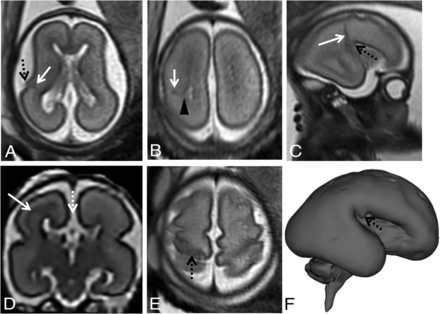
Example of associated partial CCA (white dashed arrow, D) in fetuses at 24 gestational weeks (A and B), 26 gestational weeks (C, D, F), and 31 gestational weeks (E). T2-weighted single-shot FSE images in the axial (A, B, E) and sagittal planes (C) and super-resolution 3D reconstruction of T2-weighted spin-echo FSE images through the coronal plane (D) and cortical surface (F). There is a malformation of cortical development with an abnormal “bump” in the insular region (dashed black arrow, A, C, F) and abnormal gyration in the posterior frontal cortex (dashed black arrow, E). There is a concurrent signal abnormality with low T2 signal intensity that follows the intermediate zone/subplate limit on the left (white arrow, A–D), also reaching the ventricular lining (black arrowhead, B), with slight ectasia of the homolateral posterior aspect of the lateral ventricle.
DISCUSSION
This recently developed fetal MR neuroimaging score outperforms the current oversimplified categorization of isolated CCA in terms of prognostication. When one exclusively uses the current criterion standard of excluding associated disorders, 26.2% of children had some degree of developmental delay in the iCCA group, in accordance with most literature available on the subject.5,6,8,9,20 By applying this systematic evaluation of specific brain regions, we improved the diagnostic power of MR imaging by correctly stratifying high- and low-risk cases in 90.7%. The score accuracy was higher in the normally developing group, in which 96.8% (30/31) of prognostications were correct, with the incorrect case with a borderline score of 4. In patients with some degree of neurodevelopmental delay, there were 3 incorrect classifications, all of which presented with a mild delay and MR imaging scores of 3, close to the threshold.
The MR imaging scoring system has been derived from a variety of previous structural neuroimaging findings known to be associated with CCA. Given the complexity of human brain development, the score is based on the principle that morphologic assessment of interhemispheric connectivity alone is not sufficient to offer an appropriate prognostic counseling in cases of CCA.
Despite the difference in experience in fetal MR imaging interpretation, interrater agreement was 0.98. This is similar to our previously published findings when comparing the ratings of 2 experienced raters.12
There was a significant difference in the MR imaging scores between isolated and associated CCA (P < .001), detecting a higher degree of deviation from normal brain development in aCCA despite being primarily de-signed for the specific assessment of cases classified as iCCA.12 Application of the MR imaging score did not, however, help stratify the risk in aCCA. It does not account for severe or even life-threatening extra-CNS malformations. There was also a low number of surviving children in this group with known outcomes, of which 4 patients (4/9) are developing within normal ranges, though we have a limited maximum follow-up period of 7.5 years. It can be argued that these results are skewed because minor associated anomalies are more likely to be positively counseled and not terminated, but the 4 children mentioned presented with associated CNS anomalies (MCD in 3 cases and germinolytic cyst in 1). In our opinion, this highlights the need to better understand this entity. The simplified categorization into isolated and associated does not do justice to the complex neurobiology behind this malformation.21,22
As would be expected, parents opted for TOP more often when fetuses presented with multiple malformations (aCCA: TOP, n = 24/43 [55.8%]; iCCA: TOP, n = 7/63 [9.6%]), because neurodevelopmental outcome is expected to be poor in most of these children, particularly taking into consideration that most of our associated findings were related to other CNS anomalies (90.6%). An interesting finding in our cohort was that parents opted for TOP more often when presented with a fetus with complete iCCA (n = 7/32 [21.9%]) than in cases of partial iCCA (n = 0/21), despite extensive literature proving the lack of difference in outcomes between these groups.20,23 We could not determine whether this choice related to the personal beliefs of the parents alone or to the prenatal counseling received by a variety of medical professionals (within and outside our center). In our cohort, we found no statistical difference in the neurodevelopmental outcome (P = .20), area of deficits (P = .89), need for therapy (physiotherapy, P = .40; occupational, P = .55; speech, P = 1.0), or schooling (P = .87) between partial and complete iCCA. This finding is in accordance with previous literature,8,23,24 as is the percentage of children with iCCA and some degree of developmental delay (26.2%).20,25 Furthermore, these data support the notion that counseling in cases of CCA requires further refinement and updates concerning recent genetic, morphologic, and functional neurobiologic insights into this condition. Also, in the future, more complex imaging techniques such as diffusion tensor imaging may further optimize the assessment of fetuses with CCA.
The retrospective nature of the data collection has inherent limitations. Furthermore, a large group of patients was lost to follow-up due to the tertiary referral nature of our center, with most patients coming from outside clinics or hospitals and often from foreign countries, making it challenging to obtain outcome data. Furthermore, particularly in the associated group, more than half of the pregnancies were terminated, leaving a small surviving aCCA group and limiting the intergroup comparisons. However, this information is still relevant in relation to the outcome of these pregnancies. There was also a relatively inhomogeneous assessment of outcome. Twenty-one of the iCCAs had detailed in-person neurodevelopmental assessments, while the remaining children were assessed via structured telephone interview, which can be less precise. To further validate our data and indirectly evaluate the neurodevelopmental status of the children, we also collected data on schooling (level for age and need for special schooling) as well as any specific therapy attended.
CONCLUSIONS
Despite increasing the ability to stratify the risk of neurodevelopmental delay of these patients, the MR imaging score does not substitute in any way for other investigations, namely genetic studies, which were not uniformly available in our cohort and hence were not discussed in detail. It is established that some genetic mutations are associated with a poorer prognosis, while others, such as deleted in colorectal cancer (DCC) gene, usually have a mild course.26 However, we should aim to improve our diagnostic accuracy on fetal MR imaging, independent of other studies that may or may not be available. It is the last imaging resource in prenatal diagnosis, and it should add useful information for parents and counseling whenever possible.
Footnotes
Mariana C. Diogo and Sarah Glatter were partially funded by the Austrian Science Fund, grant I 3925-B27.
Preliminary results of this paper were previously presented at: Annual Meeting of the European Congress of Magnetic Resonance in Neuropediatrics, February 26–29, 2020; Marseille, France.
Disclosures: Gregor Kasprian—RELATED: Grant: Austrian Science Fund, Comments: Joint Project Grant, role of Micro-Ribonucleic acid (mRNA) in Fetal alcohol spectrum disorders (FASD).* Mariana C. Diogo—RELATED: Grant: Austrian Science Fund*; UNRELATED: Employment: Instituto de Telemedicina, Comments: neuroradiologist. Sarah Glatter—RELATED: Grant: Austrian Science Fund 3925-B27*; Support for Travel to Meetings for the Study or Other Purposes: Medical University of Vienna. *Money paid to the institution.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received August 5, 2020.
- Accepted after revision November 10, 2020.
- © 2021 by American Journal of Neuroradiology