Abstract
BACKGROUND AND PURPOSE: MPRAGE is the most commonly used sequence for high-resolution 3D T1-weighted imaging in pediatric patients. However, its longer scan time is a major drawback because pediatric patients are prone to motion and frequently require sedation. This study compared nonenhanced accelerated MPRAGE using wave-controlled aliasing in parallel imaging (wave-T1-MPRAGE) with standard MPRAGE in infants.
MATERIALS AND METHODS: We retrospectively evaluated 68 infants (mean age, 1.78 [SD. 1.70] months) who underwent nonenhanced standard and wave-T1-MPRAGE. Two neuroradiologists independently assessed each image for image quality, artifacts, myelination degree, and anatomic delineation using the 4-point Likert scale. For diagnostic performance, both observers determined whether nonenhancing lesions were present in the brain parenchyma in 2 types of nonenhanced MPRAGE sequences.
RESULTS: Wave-T1-MPRAGE showed a significantly lower mean score and lower interobserver agreement for overall image quality and anatomic delineation than standard MPRAGE (P< .001 for each). However, there were no significant differences between the 2 types of MPRAGE sequences for motion artifacts (P = .90 for observer 1, P = .14 for observer 2) and degree of myelination (P = .16 for observer 1, P = .32 for observer 2). Among the nonenhancing pathologic lesions observed on standard MPRAGE by both observers, only 2 were missed on wave-T1-MPRAGE, and they were very tiny, faint, nonhemorrhagic WM injuries.
CONCLUSIONS: Although wave-T1-MPRAGE showed lower overall image quality than standard MPRAGE, the diagnostic performance for nonenhancing parenchymal lesions was comparable. Wave-T1-MPRAGE could be an alternative for diagnosing intracranial lesions in infants, with marked scan time reduction.
ABBREVIATIONS:
- wave-T1-MPRAGE
- MPRAGE using wave-controlled aliasing in parallel imaging
- wave-CAIPI
- wave-controlled aliasing in parallel imaging
The MPRAGE sequence is a widely used sequence for acquisition of 3D data sets of the pediatric brain because it provides high contrast between gray and white matter along with excellent spatial resolution.1 However, it requires a long scan time due to the large amount of k-space encoding and added T1 required to achieve the prepared T1-weighted contrast. It is susceptible to motion artifacts because the conventional Cartesian sampling and long scan time increase the possibility of patient movement and anxiety. Scan-time reduction is particularly important for pediatric brain imaging because it can reduce the need for sedation before performing MR imaging as well as decrease the chance of motion artifacts.2
Recently, various kinds of accelerated MR imaging techniques3⇓⇓⇓-7 have been applied to adult brain imaging for achieving scan-time reduction; however, only a few have been applied to pediatric brain imaging.2,8,9 Synthetic MR imaging enables the reconstruction of multiple synthetic sequences by simultaneous quantification of T1/T2 relaxation times and proton density for achieving whole-brain coverage from a single scan. No significant difference was found between synthetic and conventional images in the evaluation of image quality and artifacts in neonatal brain imaging.10 Wave-controlled aliasing in parallel imaging (wave-CAIPI; Siemens) is a kind of advanced parallel imaging technique that combines a corkscrew gradient trajectory with CAIPI shifts in the ky and kz directions to ensure efficient encoding of k-space along with an even spread of the voxel aliasing in all dimensions. It has been applied to various MR images and has been proved to have relatively preserved image quality and scan-time reduction compared with standard MR imaging.7,8,11,12
There are only a few studies on the application of wave-CAIPI to MPRAGE in pediatric patients.11,13 However, to our knowledge, no study has evaluated the clinical feasibility of nonenhanced MPRAGE using wave-CAIPI (wave-T1-MPRAGE) in infants. Because infants have a different condition during MR imaging and different brain tissue contrast compared with adults, application of pediatric neuroimaging (especially for the neonatal brain) can be more difficult than in adults. Thus, the purpose of our study was to compare the overall diagnostic image quality of standard MPRAGE and wave-T1-MPRAGE in infants.
MATERIALS AND METHODS
Patients
This study was approved by the institutional review board of our hospital and informed consent was not required for reviewing the images and records due to the retrospective nature of the study. We retrospectively reviewed the database and identified consecutive pediatric patients who underwent brain MR imaging between July 2021 and April 2022. The inclusion criteria of this study were consecutive patients who underwent standard- and wave-T1-MPRAGE in the same imaging session and were younger than 1 year of age. A total of 68 infants (29 boys, 39 girls; 1.78 [SD, 1.70] months of age; range, 0–9 months) were included in this study. The reasons for brain MR imaging were prematurity (57/68, 83.8%), microcephaly (4/68, 5.89%), seizure (2/68, 2.94%), fatal asphyxia (2/68, 2.94%), nystagmus (2/68, 2.94%), and prenatal ventriculomegaly (1/68, 1.47%). All patients were sedated with an oral sedative (chloral hydrate; Pocral syrup 10% mL).
Image Acquisition
All studies were performed using a 3T MR imaging scanner (Magnetom Skyra; Siemens) with a 20-channel head coil. Detailed scan parameters of standard MPRAGE and wave-T1-MPRAGE are described in the Table. In addition to the standard- and wave-T1-MPRAGE, a standard MR imaging sequence was obtained with axial FLAIR, T2-weighted, gradient-echo images. The order of the sequences was the following: wave-T1-MPRAGE followed by standard MPRAGE in 65 patients and standard MPRAGE followed by wave-T1-MPRAGE in 3 patients.
Image parameters of standard MPRAGE and wave-T1-MPRAGE
Image Analysis
Two neuroradiologists, one with 8 years of experience and the other with 2 years of experience in neuroimaging, independently reviewed all the standard MPRAGE and wave-T1-MPRAGE images using the PACS. They were blinded to the clinical information to minimize bias. Each MPRAGE sequence was reviewed at 2 different time points with at least a 4-week interval to avoid recall bias. The image quality was graded according to the following criteria: 1) overall image quality, 2) motion artifacts, 3) degree of myelination, 4) differentiation of GM-WM at the level of the lateral ventricles, 5) demarcation of the basal ganglia, and 6) demarcation of the cerebral sulci. Overall image quality was graded using the 4-point Likert scale: 1, inadequate (not acceptable for diagnostic use); 2, sufficient (acceptable for diagnostic use but with minor issues); 3, good (acceptable for diagnostic use); and 4, excellent (acceptable for diagnostic use). Motion artifacts were also graded using a 4-point grading system: 1, severe artifacts (not acceptable for diagnostic use); 2, moderate artifacts (sufficient for diagnostic use but with minor issues); 3, mild artifacts (acceptable for diagnostic use because minor artifacts do not adversely affect diagnostic use); and 4, images do not contain visible artifacts (acceptable for diagnostic use). The degree of myelination14 was assessed by the signal intensity of myelination and graded using a 4-point grading system in comparison with the adjacent GM: 1, low signal; 2, isosignal; 3, slightly high signal; and 4, prominent high signal intensity. Each criterion for structural demarcation was graded using the 4-point Likert scale: 1, not visible; 2, detectable (subtle differentiation from the neighboring structures); 3, easily delineated (easily differentiated from the neighboring structures); and 4, excellent delineation.
To evaluate the diagnostic performance, we determined whether nonenhancing pathologic lesions were present in the brain parenchyma in both MPRAGE sequences.
Statistical Analysis
The image-quality assessments of nonenhanced standard MPRAGE and wave-T1-MPRAGE were assigned numeric values. We summarized the readers’ ratings for each MPRAGE sequence and described it as mean (SD). The Wilcoxon signed-rank test was used to compare the mean values of the readers’ grading, and the McNemar test for evaluating the presence of pathologic lesions. Interobserver agreement between the 2 readers was calculated by weighted κ statistics; 0–0.20, 0.21–0.40, 0.41–0.60, 0.61–0.80, and 0.81–1.00 were regarded as slight, fair, moderate, substantial, and almost in perfect agreement, respectively, based on the Landis and Koch method.14 All statistical analyses were performed using statistical software packages (SPSS, Version 26.0, IBM; MedCalc, Version 19.8, MedCalc Software), and P value < .05 was considered statistically significant.
RESULTS
Although the overall image quality of nonenhanced wave-T1-MPRAGE was significantly lower than that of standard MPRAGE for both observers (P < .001 for each observer) (Online Supplemental Data), Wave-T1-MPRAGE showed sufficient-to-excellent image quality with a score of >2, except in 1 patient who had poor image quality due to severe motion artifacts and was scored 1 by observer 1. Nonenhanced wave-T1-MPRAGE also demonstrated significantly poor differentiation of GM-WM as well as demarcation of the basal ganglia and cerebral sulci compared with standard MPRAGE for both observers (all, P < .001 for all parameters and for both observers). Interobserver agreement of nonenhanced wave-T1-MPRAGE was significantly lower than that of standard MPRAGE (0.516 versus 0.735 for differentiation of GM-WM; 0.445 versus 0.538 for demarcation of the basal ganglia; and 0.425 versus 0.734 for demarcation of the cerebral sulci). However, motion artifacts and the degree of myelination were not significantly different between the 2 sequences for both observers (all, P > .05 for all parameters and for each observer). Furthermore, the degree of myelination had almost perfect agreement between nonenhanced standard MPRAGE and wave-T1-MPRAGE for both observers (0.885 for observer one, 0.916 for observer 2) (P < .001 for each observer).
Of the 68 patients, 19 patients (27.9%) showed nonenhancing lesions on standard MPRAGE images, including germinal matrix hemorrhage (6/19, 31.6%), hemorrhagic and nonhemorrhagic WM injuries (5/19, 26.3%), various types of hemorrhage (2/19, 10.5%), germinal matrix hemorrhage with intraventricular hemorrhage (1/19, 5.26%), periventricular leukomalacia (1/19, 5.26%), congenital anomaly (corpus callosum agenesis) (1/19, 5.26%), conatal cyst (1/19, 5.26%), parenchymal atrophy (1/19, 5.26%), and intraventricular hemorrhage (1/19, 5.26%) (Figs 1 and 2). Each observer missed 1 nonenhancing lesion on nonenhanced wave-T1-MPRAGE, and the 2 lesions were tiny, nonenhancing hyperintensities in the cerebral WM (Fig 3). Visualization of pathologic, nonenhancing lesions was not significantly different between wave-T1-MPRAGE and standard MPRAGE (P = 1.000 for each observer).
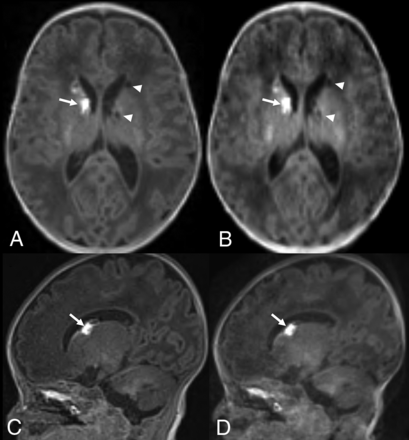
Term-equivalent-age MR imaging of a premature infant (29 weeks’ gestation). Nonenhanced standard MPRAGE images (A and C) show hyperintense germinal matrix hemorrhage (arrows) in the right caudothalamic groove and cystic changes of germinal matrix hemorrhage (arrowheads) in the left caudothalamic groove. Although wave-T1-MPRAGE images (B and D) demonstrate lower image quality than standard MPRAGE, both caudothalamic lesions are well-delineated in nonenhanced wave-T1-MPRAGE images.
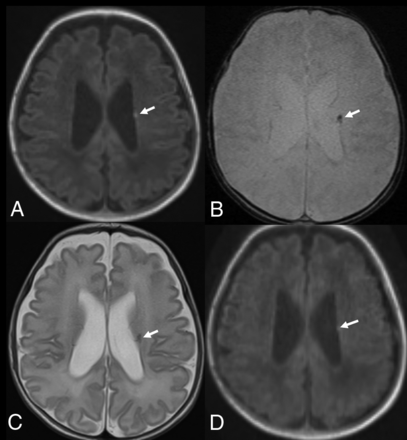
Term-equivalent-age MR imaging of a premature infant (31 weeks’ gestation). Nonenhanced standard MPRAGE (A) shows decreased cerebral WM volume and a focal T1-hyperintense lesion (arrow) at the left corona radiata. The focal corona radiata lesion (arrows) demonstrates hypointensity on gradient recalled-echo (B) and T2-weighted (C) images. Although the wave-MPRAGE image (D) shows lower image quality than standard MPRAGE, the focal T1-hyperintense lesion (arrow) at the left corona radiata is also visible in the wave-MPRAGE image.
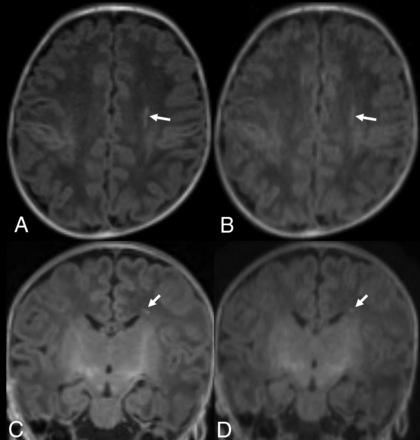
Term-equivalent-age MR imaging of a premature infant (33 weeks’ gestation). Nonenhanced standard MPRAGE images (A and C) show a focal hyperintense lesion (arrows) in the left periventricular WM. Although the wave-T1-MPRAGE images (B and D) also show the focal, hyperintense lesion (arrows) in the left periventricular WM, it is less prominently visualized than in the standard MPRAGE; thus, 1 observer missed the lesion.
DISCUSSION
In the current study, we performed the clinical evaluation of nonenhanced wave-T1-MPRAGE by assessing the overall diagnostic image quality in infants. Several studies have evaluated the clinical feasibility of wave-T1-MPRAGE;5,11,13 however, only a few studies11,13 have evaluated the clinical feasibility of wave-T1-MPRAGE in pediatric patients. To our knowledge, there has been no study on the application of nonenhanced wave-T1-MPRAGE images only for infant brain imaging. T1 and T2 properties are known to show significant changes during the first few months after birth, with a marked decrease in the brain-water content.15 In the present study, wave-T1-MPRAGE had inferior image quality and poorer anatomic demarcation than standard MPRAGE. However, there were no significant differences between wave-T1-MPRAGE and standard MPRAGE for motion artifacts, degree of myelination, and visualization of nonenhancing pathologic lesions. Furthermore, the use of wave-CAIPI reduced the acquisition time by 45% compared with standard MPRAGE (2 minutes 14 seconds versus 4 minutes 55 seconds).
Previous studies11,13 that applied wave-CAIPI to MPRAGE in pediatric brain imaging protocol also showed a marked reduction in the total scan time. Tabari et al11 used an even higher acceleration factor than that used in our study (acceleration factor: 6 or 9 versus 4) and reported that wave-T1-MPRAGE had more image noise and was less preferable for the evaluation of anatomic structures compared with standard MPRAGE. However, there were no cases in which enhancing or nonenhancing pathologic lesions were not visualized on wave-T1-MPRAGE. Their results were consistent with those of our study showing lower image quality and poor anatomic demarcation with wave-T1-MPRAGE; however, the visualization of nonenhancing pathologic lesions was not significantly different between wave-T1-MPRAGE and standard MPRAGE.
Unlike our study, Yim et al13 reported that there was no significant difference between wave-T1-MPRAGE and standard MPRAGE for the differentiation of GM-WM and demarcation of the basal ganglia and cerebral sulci. Although they applied wave-T1-MPRAGE to pediatric patients, the mean age of their study subjects was much older than that of the infants in our study (71.9 [SD, 60.8] months versus 1.78 [SD, 1.70] months); thus, this factor might account for the different study results. However, the overall image quality of wave-T1-MPRAGE was also significantly lower than that of standard MPRAGE, similar to our study. Furthermore, they showed excellent agreement between wave-T1-MPRAGE and standard MPRAGE for the detection of enhancing and nonenhancing lesions.
Lower image quality of wave-T1-MPRAGE than standard MPRAGE might be associated with more noise in wave-CAIPI images. Previous studies8,12,13 have reported that the wave-CAIPI images showed lower SNR in the central coil area than in the peripheral area, and decreasing SNR was associated with an increase in the acceleration factor. Future technical developments in postprocessing for denoising and image regularization could minimize the noise amplification and wave-specific blurring artifacts.
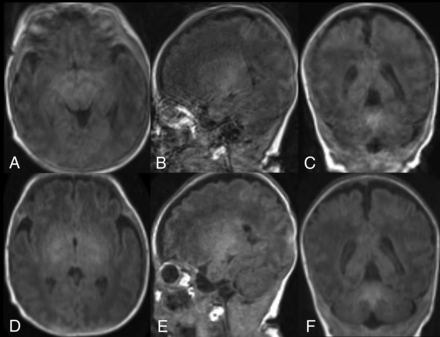
In our study, motion artifacts were not significantly different between wave-T1-MPRAGE and standard MPRAGE. However, 2 cases showed severe motion artifacts, which are not acceptable for diagnostic use on standard MPRAGE, while wave-T1-MPRAGE showed mild artifacts, which are acceptable for diagnostic use (Fig 4). Tabari et al11 also demonstrated 1 case with more severe motion artifacts on standard MPRAGE compared with wave-T1-MPRAGE. These cases show the potential for a faster scan to improve the image quality in motion-prone pediatric patients. According to previous technical studies,16,17 wave-CAIPI reduces the overall motion artifacts because it permits each average to be acquired within a shorter timeframe. Further studies are needed to validate the potential for a faster scan to improve the image quality.
Brain MR imaging of a premature infant (29 weeks’ gestation) 1 month after birth. Nonenhanced standard MPRAGE images (A–C) show severe motion artifacts, which are not acceptable for diagnostic use, while wave-T1-MPRAGE (D–F) shows mild motion artifacts, which are acceptable for diagnostic use.
This study has several limitations. First, it has an inevitable selection bias due to its retrospective nature. Future multicenter studies evaluating the clinical feasibility of nonenhanced wave-T1-MPRAGE in a larger number of infants are required for validating our results. Second, we could not randomize the order of wave-T1-MPRAGE and standard MPRAGE because of the retrospective design of the study. Thus, the motion artifacts of wave-T1-MPRAGE might be underestimated in this study. Future studies with a randomized order of image acquisition are needed to validate the results. Third, we could not perform quantitative analyses for the image quality. Because we performed wave-T1-MPRAGE in infants younger than 1 year of age, we could not perform quantitative analyses such as calculating the contrast-to-noise ratio and contrast ratio between the gray-white matter in the 2 types of T1-MPRAGE sequences. Furthermore, we could not perform brain tissue segmentation using commercial software. We tried to measure it automatically using NeuroQuant software (CorTechs Labs), but it was not successful. Because myelination is incomplete and the FOV is relatively small in the infant period and infants are vulnerable to movement, brain tissue segmentation is more difficult compared with that in older pediatric patients.18,19
CONCLUSIONS
Although nonenhanced wave-T1-MPRAGE showed lower overall image quality and anatomic demarcation than standard MPRAGE, the diagnostic performance for the presence of nonenhancing parenchymal lesions was comparable with that of standard MPRAGE. Therefore, nonenhanced wave-T1-MPRAGE could be an alternative method for diagnosing intracranial lesions in infants, with the advantage of marked reduction in the scan time.
Footnotes
This work was supported by 2022 Inje University Busan Paik Hospital research grant.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received June 16, 2022.
- Accepted after revision September 19, 2022.
- © 2022 by American Journal of Neuroradiology