Abstract
BACKGROUND AND PURPOSE: Knowledge of anatomic markers of the hand motor cortex is essential in the evaluation and treatment of motor neurologic diseases for both adults and developing populations. However, hand motor cortex variants in developing brains remain to be investigated. Our objective was to observe morphologic variants of the hand motor cortex in developing brains from neonates through childhood.
MATERIALS AND METHODS: In this study, 542 participants (0∼15 years of age) were retrospectively enrolled and divided into different age groups. The hand motor cortex morphology was evaluated on the basis of 3D T1WI. Variations in hand motor cortex variants were compared among different age groups. Inter-gender and interhemispheric differences of hand motor cortex variants were also evaluated.
RESULTS: Various hand motor cortex variants could be observed in developing brains, even in the neonatal period. One new morphologic shape, “immature Ω,” was found in neonates and infants. The proportion of this new shape decreased dramatically during the first year after birth, then disappeared after 1 year of age. It persisted for a longer time in the right hemisphere and in males. However, sex or hemispheric effects on the distribution of the proportion of variants were not statistically significant. Furthermore, the proportion of concordance of the bilateral hand motor cortex showed an increasing trend with age (P = .006), higher in females than males.
CONCLUSIONS: Various hand motor cortex variants already existed at birth. The distribution of proportions of different variants developmentally varied during the first year after birth and became stable after 1 year of age. The concordance of the bilateral hand motor cortex could be influenced by age and sex.
ABBREVIATIONS:
- ACPC
- anterior/posterior commissure
- GA
- gestational age
- HMC
- hand motor cortex
- PMA
- postmenstrual age
The hand motor cortex (HMC) has been identified as a knob on the precentral gyrus.1 It is always the recognition and stimulation/treatment target in the evaluation and treatment of neurologic motor diseases (eg, stroke,2⇓⇓⇓-6 focal hand dystonia,7 and amyotrophic lateral sclerosis8) or glioma.9 Furthermore, it is an identifiable neuroanatomic structure for delineating the corticospinal tract.10 Therefore, the identification of the HMC is essentially important for the exploration of the mechanism of handedness,11 hand motor skills,12 and eye-hand coordination.13
MR imaging provides a nonradiative tool for investigating the morphology of the HMC in vivo. The previous known shape of the HMC is a typical hook14,15 on the MR imaging sagittal plane, and a V-shaped anatomic signature16 and an Ω on the axial plane.1 The Ω sign was gradually recognized as a reliable landmark for the localization of the HMC.16 Furthermore, 5 morphologic variants (Ω, medially asymmetric ε, ε, laterally asymmetric ε, and null) of the HMC have been recognized on MR imaging in adults.15 Knowledge of anatomic markers of the HMC on MR imaging is essential in research and clinical practice, not only for adults but also for the developing population. Specifically, the treatment target of transcranial magnetic stimulation was always placed on the primary motor cortex in children with spastic cerebral palsy with hand dysfunction.17,18 For patients with brain tumors, the width and height of the HMC and the distance from the tumor to the HMC on MR imaging are related to neurologic motor deficits.9 Although HMC variants have been recognized in adults,15 the morphology of the HMC in children may be different from that in adults because the brain develops dramatically during early childhood.19,20 However, little information has been reported about the characteristics of the HMC on the developing population, and the effects of age and sex on HMC morphology remain to be assessed. Therefore, it is necessary to investigate the morphology of the HMC in developing brains.
On the basis of the above considerations, this study tried to use MR imaging to investigate the morphologic variants of the HMC in developing brains from neonates through childhood. HMC variants were compared among different age groups. Sex and hemisphere effects on HMC variants were also evaluated. Furthermore, the concordance of the bilateral HMC was analyzed.
MATERIALS AND METHODS
This retrospective study was approved by the institutional review board of the First Affiliated Hospital of Xi’an Jiaotong University. Written informed consent was obtained from parents or guardians of participants.
Subjects
Subjects were enrolled consecutively from August 2012 to December 2019. According to the postnatal age at MR imaging, the enrolled subjects were divided into neonates (age, ≤28 days), infants (>28 days to <1 year), toddlers (1 to <3 years), preschool children (3 to <6 years), school-age children (6 to <10 years), and adolescents (10 to <15 years). To reveal more detailed alterations during the neonatal period, we divided neonates into preterm (gestational age [GA], < 37 weeks) and term (GA, ≥37 weeks) groups. All infants, toddlers, preschool children, school-age children, and adolescents were term birth. Because of the difference in the disease spectrum between the neonate group and other age groups, we performed the inclusion and exclusion using different criteria.
The detailed inclusion and exclusion criteria were as follows:
Inclusion criteria: neonate group: age at MR imaging, ≤ 28 days. Other age groups: 1) GA ≥37 weeks; 2) age at MR imaging, 29 days∼15 years of age.
Exclusion criteria: 1) abnormal findings on MR imaging or diseases influencing the brain maturation: neonatal group: punctate white matter lesions, metabolic disorders, intracranial hemorrhage, periventricular leukomalacia, congenital malformations of nervous system, hydrocephalus, small for GA, hypoxic-ischemic encephalopathy, and intracranial infection. Other age groups: focal white matter hyperintensity on T2 FLAIR, enlargement of subarachnoid spaces, dilated Virchow-Robin spaces, encephalomalacia, periventricular leukomalacia, congenital malformations of the nervous system, hydrocephalus, malformation of cerebral vessels, metabolic disorders, and others including cerebral trauma and intracranial tumor, epilepsy, febrile convulsion, tic disorder, visual abnormalities, intracranial infection, hypoxic-ischemic encephalopathy, and mental, behavioral, or neurodevelopmental disorders; 2) incomplete MR images or clinical information (including perinatal characteristics and clinical conditions).
Data Acquisition
MR images were acquired using a 3T scanner (Signa HDxt; GE Healthcare) with an 8-channel head coil. 3D fast spoiled gradient recalled-echo T1WI was performed using the following parameters: TR/TE, 10.42/4.74 ms; isotropic resolution, 1 × 1×1 mm3; matrix, 240 × 240; thickness, 1 mm; FOV, 240 × 240 mm2.
Image Processing
The 3D T1WIs of the cerebrum were translated and rotated into the anterior/posterior commissure (ACPC) plane. In adults, the HMC was located at Talairach coordinates of x = ±34, y = −29, z = 50.15,21 Due to the developmental changes of brain morphology, the optimal level for evaluating the HMC was different across individuals. Here, the ACPC plane was defined as the zero point of the z-axis. Then, the evaluation plane above the ACPC line was located at different coordinates in different age groups: neonates, 32 (SD, 3) mm; infants, 39 (SD, 4) mm; toddlers, 46 (SD, 4) mm; preschool children, 49 (SD, 4) mm; school-age children, 50 (SD, 3) mm; and adolescents 50 (SD, 3) mm.
Morphologic Variant Classification of the HMC
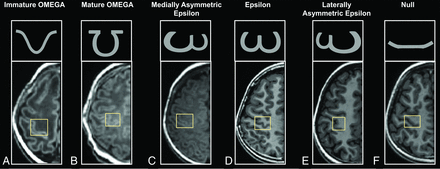
Five different morphologic variants have been found for the adult HMC: Ω, medially asymmetric ε, ε, laterally asymmetric ε, and null.15 Besides these variants, this work revealed 1 new shape like but different from the Ω type. Because this shape could be found in neonates and infants and disappeared from the toddler period on, this work termed it “immature Ω” (Fig 1). The identification of the immature Ω was performed according to the following appearances: The shape was sloped; the outside angle between any side of the HMC and the base side was >90°; the ratio between the height of the HMC and thickness of gray matter was ≥ 4; and the ratio between the height and width of the HMC was ≤1. For comparison, the HMC with an outside angle of ≤90°, the ratio between the height of the HMC and thickness of gray matter was ≥ 4, or the Ω type was classified as the “mature Ω.” Two radiologists (with >5 years’ experience in the interpretation of the pediatric brain MR imaging), blinded to the demographic information of participants, independently evaluated the HMC type, and one of them preformed the evaluation twice. Moreover, disagreements across different evaluations were resolved by discussion with a senior radiologist (>15 years’ experience).
Appearances of HMC variances. Immature Ω (A): preterm neonate (female, GA = 32+1 weeks, PMA = 32+5 weeks). Mature Ω (B): term neonate (female, GA = 38+2 weeks, PMA = 38+6 weeks). Medially asymmetric ε (C): infant (female, GA = 39+1 weeks, age = 1.18 months). ε (D): adolescent (female, GA = 42 weeks, age = 10.36 years). Laterally asymmetric ε (E): school-age children (female, GA = 39+6 weeks, age = 8.37 years). Null (F): preschool children (male, GA = 40 weeks, age = 5.96 years).
Statistical Analysis
Statistical analyses were performed using SPSS (Version 18; IBM). Two neuroradiologists independently evaluated the HMC. Intra- and interobserver reliability was evaluated using the κ test. Categoric variables (the distribution of the HMC variant in different age groups) are shown as percentages and percentages in stacked bar charts. Categoric variables were compared using the Monte Carlo method test. P < .05 was considered statistically significant. In multiple comparisons, P < .0024 (.05/21) was considered statistically significant after the Bonferroni correction. The χ2 test for trend was performed for the proportion of concordance of the bilateral HMC variants in each age group.
RESULTS
Participants
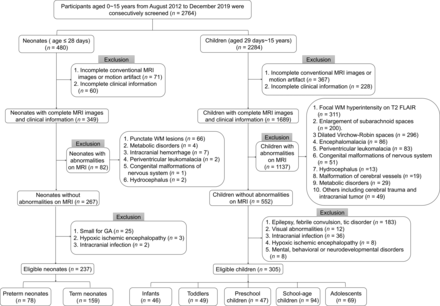
According to the inclusion and exclusion criteria, this work enrolled 542 participants, including 78 preterm neonates (40 males), 159 term neonates (103 males), 46 infants (32 males), 49 toddlers (33 males), 47 preschool children (34 males), 94 school-age children (53 males), and 69 adolescents (41 males) (Fig 2). No significant differences in GA, postnatal age, or postmenstrual age (PMA) at MR imaging were found between males and females in each age group (P > .05).
Flow chart of the inclusion and exclusion criteria.
Intra- and Interobserver Reliability
The intraobserver and interobserver agreement for the HMC-type classification was 93.91% (κ value = 0.89; standard error = 0.02) and 90.59% (κ value = 0.84; standard error = 0.02), respectively.
Percentage of Morphologic Variants in Different Age Groups
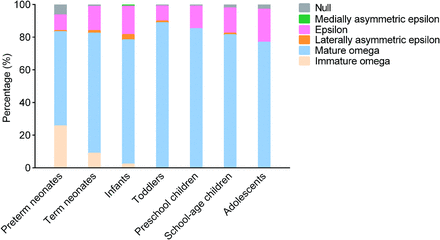
In total, 6 variants (including 5 conventional variants and the immature Ω) could be found in participants. The sequence of the percentage of 542 participants (1084 HMCs) in descending order was the following: mature Ω (75.65%), ε (14.49%), immature Ω (6.46%), null (2.21%), laterally asymmetric ε (1.11%), and medially asymmetric ε (0.09%). The proportions of HMC variants in different age groups are shown in Fig 3.
The proportional distribution of HMC variants in different age groups.
The percentage distribution of HMC variants was statistically different across age groups. In the pair-wise comparison across 7 age groups (Online Supplemental Data), there were significant differences in the distribution of the HMC variant between the preterm neonates and other age groups (P < .0024). The mature Ω showed the trend of “increasing, relatively stable.” Except for neonates, there was no significant difference in the distribution of the HMC variants among other age groups (P > .0024). Specifically, the proportion of the immature Ω HMC decreased from 25.64% in preterm neonates to 8.81% in term neonates, 2.17% in infants, and 0% in toddlers. The proportion of the mature Ω HMC increased from 57.69% in preterm neonates to 73.58% in term neonates and 76.09% in infants and was stable from toddler age on at 76.81%∼88.78% (median, 83.25%). The proportion of the ε (9.18%∼20.29%; median, 14.78%), the laterally asymmetric ε (0%∼3.26%; median, 1.02%), the medially asymmetric ε (0%∼1.09%; median, 0%), and the null HMC (0%∼6.41%; median, 1.26%) did not differ significantly among age groups.
Morphologic Variants of the HMC in Different Sexes and Hemispheres
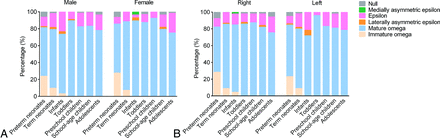
As shown in Fig 4, there was no significant difference in the distribution proportion between males and females or between left and right hemispheres in each age group (P > .05). Figure 4 also shows that the immature Ω sign seems to persist for a longer time in infants in the right hemisphere and in males.
The proportional distribution of HMC variants in different sexes (A) and different hemispheres (B).
Furthermore, the interhemispheric concordance of the HMC variants was also evaluated at the individual level. As for the same variant between the left and right hemispheres, the Ω type accounted for 97.50% (immature Ω accounted for 25%) in preterm neonates, and the null accounted for 2.50%. The Ω type accounted for 95.05% (the immature Ω accounted for 5.94%) in term neonates, the ε accounted for 3.96%, and the lateral ε accounted for 0.99%. The mature Ω was also the HMC variant with the maximum proportion in other age groups (accounting for >90%): infants (90.32%), toddlers (100%), preschool children (94.74%), school-age children (94.12%), and adolescents (91.30%). The proportion of the interhemispheric concordance showed an increasing trend with age (χ2 = 7.540, P = .006).
As for different sexes, the ratios of participants with the same bilateral HMC variant were ≥50% in both males and females (Table). The consistency ratio of females was higher than in males in most age groups.
The proportion of concordance of the HMC variant in bilateral hemispheres in males and females of different age groups
DISCUSSION
This study demonstrated morphologic variants of the HMC in developing brains from neonates through childhood. Besides the previously proposed 5 variants of the HMC in adults,1,15 we classified the Ω into 2 subtypes: mature Ω and immature Ω. Results demonstrated that various HMC variants already existed at birth and the proportional distribution of variants changed dramatically during the first year after birth. The concordance of the bilateral HMC showed an increasing trend with age. It could also be influenced by sex. Results here may provide a reference for the future investigations of the influence of pediatric diseases on the HMC.
HMC Morphologic Variants and Brain Development
Results here revealed that various HMC variants already existed in neonates. The immature Ω type accounted for a noticeable proportion. Typically, the central sulcus forms at a GA of 20–23 weeks, while the precentral gyrus forms at a GA of 24–27 weeks, and the secondary cerebral gyrus, at a GA of 32–35 weeks.22,23 In this study, the PMA at MR imaging of all neonates was >32 weeks. Therefore, the HMC in the immature state could be observed in neonates here. This study also found that the distribution of proportions in different variants of the HMC dramatically changed with age, especially before 1 year of age. The proportion of the immature HMC decreased, while the mature HMC increased. This observation may be because the cortex is still in a continuous folding process. During the third trimester of gestation, cortical folding changes dramatically in the brain.24 A previous study observed that the synapse formation and the synapse pruning begin around a GA of 20 weeks and the myelin formation begins around a GA from 30 to 32 weeks.25 These observations suggest that changes of the HMC morphology may also be associated with the ongoing myelin sheath and synaptic remodeling processes.20,26 It has been proposed that the tension-mediated folding increases as the axons strongly pull the interconnected regions together.27 Development of axons underlying the central sulcus may cause the morphology of the HMC to change toward the mature type.
In addition, the cortical surface area of the precentral gyrus continues to increase after 1 year of age,20,28 while the sulci depth remains stable.29 Therefore, the change of the precentral gyrus after 1 year of age here mainly reflects the increase of the gyrus width. Meanwhile, spatial distributions of the sulcal pits (the locally deepest points in sulci) show high consistency between infants and adults.30 In agreement with these cortical maturation characteristics, the proportional distribution in the HMC variants reaches a relatively stable state after 1 year of age.
Potential Influencing Factors on HMC Morphologic Variants
HMC variants in adults have demonstrated that the proportions of sequences from high to low are as follows: Ω, ε, laterally asymmetric ε, medially asymmetric ε, and null.15 In this study, the distribution of the mature Ω type in adolescents was similar to that of adults. Meanwhile, there were also differences: The ratio of ε in the current work was higher than that of adults, and the ratios of laterally asymmetric ε and medially asymmetric ε were lower. This finding may be due to a combination of factors of ethnic, genetic, customary, and environmental differences during crucial developmental stages of the brain. Previous studies have shown that the primary motor cortex in higher primates has internal subdivisions in the rostrocaudal direction and in 2 sectors connected to the spinal cord.31,32
Additionally, in a direct electrophysiologic study of humans, the anatomo-functional subdivisions of the HMC include 2 sectors: the caudal one and the rostral one, playing different roles in motor control.33 The caudal HMC is the most excitable sector. The rostral HMC is a crucial area for shaping functional synergies for hand-object interaction. These findings suggest that the human hand knob is an anatomo-functional heterogeneous region organized along a motor-cognitive gradient.34 Thus, we speculated that the position of the middle fissure of ε in morphology may be related to these 2 sectors in the anatomo-functional subdivision. In families of the enrolled participants, early fine-motor skills of manipulating chopsticks and writing implements are expected.35 Exuberant connectivity of the brain in the early life is pruned by competition, influenced by the early experience.19 Thus, plasticity and adaptation of the early brain development could lead to differences in the HMC morphology, especially in ε, laterally asymmetric ε, and medially asymmetric ε.19
The proportion of the same HMC variant in the bilateral hemispheres was higher than that of the inconsistent HMC variants in the bilateral hemispheres. According to comparisons across preterm neonates, term neonates, infants, and toddlers, age was a potential factor influencing the concordance of the HMC variant in the bilateral hemispheres. This finding may be related to the maturation of HMC morphology. With the process of brain development and cortex folding, some of the HMC changed from the immature Ω type into the mature Ω type. This feature may be the main factor leading to the increase of the concordance of the HMC variant in the bilateral hemispheres, especially during the first year after birth. Moreover, the immature Ω sign seems to persist for a longer time on the right side. This interhemispheric difference may be associated with the lateralization of motor function during development. A previous study indicated that early brain lateralization in humans could be influenced or reinforced by the asymmetric posture of the developing fetus.36 From 14 weeks after conception, fetuses preferentially turn their heads to the right and suck on their right thumbs; this preference appears to be maintained throughout pregnancy.37 In addition, most fetuses are in a head-down orientation on the mother’s left side, a preferred position during the final trimester, favoring movement of the right arm by providing more space on the right side, thus contributing to the hand motor lateralization.36,38 Thus, early increased movement of the right hand may promote the folding and maturation of the left hemisphere HMC. Meanwhile, the neonatal left precentral gyrus has more efficient communications than the right homolog.39 After delivery and when in a supine position, most neonates prefer to lie with their heads turned to the right, resulting in greater perceptual experience related to the right hand.40 Therefore, a shorter persisting time of the immature Ω type in the left hemisphere may be partly a result of the adaption to lateralized functional needs at birth.
Furthermore, the proportion of consistent HMC variants in the bilateral hemispheres was higher in females than that in males. According to a previous study, an interhemispheric concordance for HMC variants was observed only in females.15 Several studies on brain asymmetry suggested that the male brain may be more lateralized or asymmetric than the female brain.41⇓-43 In an animal study, it was also observed that preventing the flow of androgens from the testis to the brain blocks the formation of the normal rightward brain asymmetry in male rats. Similarly, the female pattern can be reversed to the male pattern by neonatal ovariectomy.44
These findings suggest that levels of androgenic and ovarian steroids play a part in regulating brain asymmetry. The growth of children is accompanied by changes in the levels of endocrine hormones, including sex hormones in females and males.45 Therefore, sex may be another potential influencing factor on the concordance of the HMC variant in the bilateral hemispheres. Moreover, it has been found that males have smaller fractional anisotropy and larger axial diffusivity than females from birth to 2 years of age.46 This finding suggests that the motor tract development in males is slightly behind that in females.46 Meanwhile, the tension-mediated folding increases as axons strongly pull the interconnected regions together.27 Therefore, a longer persisting time of the immature Ω type in males may be partly a result of the inter-gender difference in the motor-related white matter maturation.
Limitations
There are several limitations. First, although we included a large sample of subjects 0–15 years of age, samples were from 1 single center. Moreover, not all participants were recruited from healthy families. Participants were enrolled partly from the those with healthy findings on examinations and subjects with clinical symptoms (details are listed in Online Supplemental Data). There may be selection bias, though no subjects had abnormalities on MR imaging. Multisite research is necessary in the future to further verify our results. Second, changes in the morphology of individuals were not followed up. Therefore, this was a cross-sectional study. Changes in the variance were solely statistical associations rather than longitudinal observations. Third, this study did not assess the HMC morphology quantitatively. The relationship between morphologic parameters of the HMC and hand function outcomes remains to be investigated in further work.
CONCLUSIONS
This study demonstrated that various HMC variants already existed at birth. The distribution of proportions of different variants developmentally varied during the first year after birth and became stable after 1 year of age. The concordance of the bilateral HMC could be influenced by age and sex.
Footnotes
Fan Wu and Huifang Zhao contributed equally to this work.
Jian Yang and Xianjun Li served as senior authors.
This work was supported by the National Natural Science Foundation of China (81971581, 81901823, 81771810, and 81901516) and the Innovation Team Project of Natural Science Fund of Shaanxi Province (2019TD-018).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received July 6, 2021.
- Accepted after revision October 20, 2021.
- © 2022 by American Journal of Neuroradiology