Abstract
BACKGROUND AND PURPOSE: Infarct evolution after endovascular treatment varies widely among patients with stroke and may be affected by baseline characteristics and procedural outcomes. Moreover, IV alteplase and endovascular treatment may influence the relationship of these factors to infarct evolution. We aimed to assess whether the infarct evolution between baseline and follow-up imaging was different for patients who received IVT and EVT versus EVT alone.
MATERIALS AND METHODS: We included patients from the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN)-NO IV trial with baseline CTP and follow-up imaging. Follow-up infarct volume was segmented on 24-hour or 1-week follow-up DWI or NCCT. Infarct evolution was defined as the follow-up lesion volume: CTP core volume. Substantial infarct growth was defined as an increase in follow-up infarct volume of >10 mL. We assessed whether infarct evolution was different for patients with IV alteplase and endovascular treatment versus endovascular treatment alone and evaluated the association of baseline characteristics and procedural outcomes with infarct evolution using multivariable regression.
RESULTS: From 228 patients with CTP results available, 145 patients had follow-up imaging and were included in our analysis. For patients with IV alteplase and endovascular treatment versus endovascular treatment alone, the baseline median CTP core volume was 17 (interquartile range = 4–35) mL versus 11 (interquartile range = 6–24) mL. The median follow-up infarct volume was 13 (interquartile range, 4–48) mL versus 17 (interquartile range = 4–50) mL. Collateral status and occlusion location were negatively associated with substantial infarct growth in patients with and without IV alteplase before endovascular treatment.
CONCLUSIONS: No statistically significant difference in infarct evolution was found in directly admitted patients who received IV alteplase and endovascular treatment within 4.5 hours of symptom onset versus patients who underwent endovascular treatment alone. Collateral status and occlusion location may be useful predictors of infarct evolution prognosis in patients eligible for IV alteplase who underwent endovascular treatment.
ABBREVIATIONS:
- EVT
- endovascular treatment
- eTICI
- expanded treatment in cerebral ischemia
- FIV
- follow-up infarct volume
- IQR
- interquartile range
- IVT
- IV alteplase
- mAOL
- modified arterial occlusive lesion
- RCT
- randomized controlled trial
Endovascular treatment (EVT) preceded by administering IV alteplase (IVT) is the current standard of care and is effective in patients with acute ischemic stroke.1
A first meta-analysis of 3 Asian randomized controlled trials (RCTs) comparing EVT alone with IVT before EVT suggested noninferiority of EVT alone.2 However, 4 following RCTs, including the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN)-NO IV trial (ISRCTN80619088), demonstrated neither superiority nor noninferiority of EVT alone with regard to functional outcome at 90 days after stroke.3⇓⇓-6 A recent expedited guideline from the European Stroke Organization and the European Society for Minimally Invasive Neurologic Therapy, a meta-analysis of all 6 RCTs, recommended IVT before EVT over EVT alone.7 While there were no large differences in clinical outcome between the overall study groups in the RCTs, individual variations in infarct evolution might still be present.5,6,8,9 These are clinically relevant because infarct evolution and infarct growth in particular are associated with functional outcome after EVT and differ from patient to patient.10⇓⇓⇓⇓⇓⇓⇓-18 The following factors affect subacute infarct evolution: collateral status, occlusion location, onset-to-reperfusion time, reperfusion rate, total attempts, and early re-occlusion of the target artery.19⇓-21 These factors may be influenced by IVT before EVT.
CTP acquisition allows quantification of the CBF to estimate the brain tissue viability and ischemic core volume on baseline imaging.22 The estimated ischemic core may still evolve in the first days to weeks after stroke onset, despite timely and adequate endovascular treatment.17,23⇓-25 To our knowledge, infarct evolution has not yet been compared between patients with endovascularly treated acute ischemic stroke who were randomized for IVT and EVT versus those with EVT alone.
In this post hoc analysis of the MR CLEAN-NO IV trial, we aimed to assess whether the infarct evolution between baseline and follow-up imaging was different for patients who received IVT and EVT versus EVT alone. Additionally, we aimed to identify which clinical and procedural outcomes are associated with infarct evolution in patients with acute ischemic stroke who received IVT and EVT versus EVT alone.
MATERIALS AND METHODS
Patient Selection
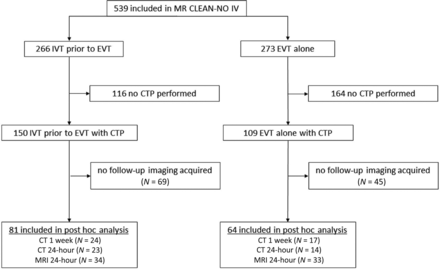
We included patients with baseline CTP and follow-up DWI or NCCT from the MR CLEAN-NO IV trial.5 The MR CLEAN-NO IV trial included patients with acute ischemic stroke due to an intracranial proximal occlusion of the anterior circulation who were directly admitted to an EVT-capable center between January 2018 and October 2020. If eligible for EVT and IVT administration within 4.5 hours, patients were randomly assigned to receive either EVT alone or IVT followed by EVT. Analyses were performed in the as-treated population. Details of the trial protocol were previously published.26 A flow chart explaining the inclusion criteria of this study is provided in Fig 1.
Flow chart of patient selection.
Image Acquisition and Postprocessing
Baseline CTP images were acquired according to site-specific baseline CT acquisition protocols. CTP data were centrally postprocessed by an independent core lab using syngo.via (Version VB40; Siemens). The ischemic core was estimated using a CBV of <1.2/100 mL, and the penumbra was estimated using a CBF of <27/100 mL/min.27 A smoothing filter (smoothing strength, 10 mm) was applied.27 Expert visual-quality assessment of the CTP results was performed by 2 experienced neuroradiologists (with >10 and >15 years of experience), and craniocaudal cropping was allowed to remove obvious artifacts at the level of the skull base.28 Follow-up imaging was acquired at a median of 24- to 48-hour DWI, 24-hour NCCT, or 5- to 7-day NCCT. DWI was the preferred technique for determining the follow-up infarct volume (FIV). If DWI was not available, follow-up NCCT was used to segment the FIV using a semiautomated segmentation method,29 with subsequent expert visual-quality assessment (>15 years of experience). In case both 24-hour and 5- to 7-day NCCT were available, the 5- to 7-day NCCT was used to assess the FIV. If hemorrhagic transformation was present, the hemorrhagic regions were included in the segmentation volume. Hemorrhagic transformation was scored by an independent core lab and defined according to the Heidelberg Bleeding Classification.30 Recanalization on follow-up imaging was assessed on either CTA or MRA using the modified arterial occlusive lesion (mAOL) score.31
Infarct Evolution and Imaging Assessment
We compared the infarct evolution and occurrence of substantial lesion growth between patients who received IVT and EVT versus patients who underwent EVT alone. Infarct evolution was calculated by subtracting the CTP core volume from the FIV. Overestimation of the FIV by CTP was defined as CTP core volume of >FIV. Substantial infarct growth was defined as an increase in FIV of >10 mL. All imaging data were assessed by an independent core laboratory of neuroradiologists or radiologists. Postprocedural reperfusion was assessed on postprocedural DSA. Successful reperfusion was defined as extended TICI (eTICI) 2b–3, and complete reperfusion was defined as eTICI 3. Recanalization of the target artery was assessed on 24-hour follow-up CTA or MRA. Incomplete patency of the target artery on follow-up imaging was defined as mAOL 0–1.32
Statistical Analysis
Baseline clinical and imaging variables were compared between patients with IVT prior to EVT versus EVT alone using the Mann-Whitney U or χ2 test. The primary outcome in this study was infarct evolution in milliliters. To assess the association of IVT before EVT with substantial infarct growth (ie, positive infarct evolution of >10 mL), we performed uni- and multivariable logistic regression analysis adjusted for the following potential confounders: ASPECTS, CTA collateral score, onset-to-reperfusion time, reperfusion rate (scored on the eTICI scale), occlusion location, total attempts, occurrence of any hemorrhagic transformation, and re-occlusion rates on follow-up CTA or MRA (scored on the mAOL scale). We checked our model for multicollinearity by determining the variance inflation factor values of all variables included in the model. Infarct evolution between patients with successful reperfusion versus unsuccessful reperfusion was compared using Mann-Whitney U tests. We performed a sensitivity analysis for patients who underwent 24-hour follow-up DWI and NCCT imaging to evaluate whether including 1-week follow-up NCCT FIVs would affect our findings. We performed a sensitivity analysis for patients with tandem lesions because tandem lesions (ie, occlusion or stenosis of the ICA with a concomitant intracranial occlusion) are known to be associated with lower reperfusion rates and, therefore, may show different infarct evolution.33 Furthermore, we explored whether our results were consistent in a subgroup of patients without hemorrhagic transformation because large hemorrhages between baseline and follow-up imaging can strongly affect the FIV assessment. Both sensitivity analyses are reported in the Online Supplemental Data.
Protocol Approval and Patient Consent
The MR CLEAN-NO IV trial protocol was approved by national central ethics committees and by research boards at each participating center. The final versions of the trial protocol and statistical analysis plan are both available at www.nejm.org. The MR CLEAN-NO IV trial was conducted in accordance with the revised Helsinki guidelines.
Data Availability
Individual patient data cannot be made available under Dutch law because we did not obtain patient approval for sharing individual patient data. All syntax files and output of statistical analyses are available on reasonable request.
RESULTS
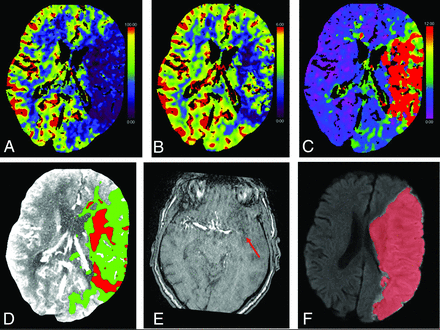
From 539 patients included in the MR CLEAN-NO IV trial, 228 had available CTP results. Of these 228 patients, follow-up imaging was performed in 145 patients, and they were included in our post hoc analysis. Eighty-one (56%) patients received IVT and EVT. Baseline characteristics such as age, sex, and baseline NIHSS were comparable for patients who received IVT and EVT versus patients who underwent EVT alone. Median baseline CTP-estimated ischemic core volume was 17 (interquartile range [IQR] = 4–35) mL versus 11 (IQR = 6–24) mL (P = .5). The median FIV was 13 (IQR = 4–48) mL versus 17 (IQR = 4–50) mL (P = 1.0). CTP ischemic core overestimation of >10 mL occurred in 17/81 (21%) versus 9/64 (14%) patients and occurred primarily in the white matter. The time between baseline CTP and follow-up imaging was comparable (27 versus 33 hours, P = .3). Good functional outcome occurred in 45/81 (56%) patients who received IVT and EVT versus in 37/64 (58%) patients who received EVT alone (OR = 0.86; 95% CI, 0.42–1.73; P = .7). Four (3%) patients showed early recanalization (ie, recanalization before EVT). Two patients with early recanalization received IVT before EVT. An example of a patient with a left-sided M1 occlusion and a baseline CTP-estimated core of 65 mL is shown in Fig 2. This patient underwent successful EVT alone (eTICI 3) with an onset-to-reperfusion time of 195 minutes. Follow-up CTA showed a visible calcified embolus in the left M1 (mAOL 0). Follow-up DWI showed substantial infarct growth (384 mL). See the Online Supplemental Data for a complete description of baseline, procedural, and outcome characteristics stratified per study subgroup.
Baseline CTP of a patient with a left-sided M1 occlusion with substantial infarct growth with complete reperfusion (eTICI 3) after 5 attempts within 195 minutes of onset. The collateral score at baseline CTA (not shown) was zero. The CBF, CBV, and time-to-maximum parameter maps are shown in panels A–C. D, Ischemic core (red) and penumbra (green) estimations. E, Follow-up MRA shows a re-occluded M1 with visible calcified embolus (red arrow, mAOL = 0). F, Follow-up DWI acquired at 15 hours after baseline imaging with FIV segmentation (red).
Association of Baseline Characteristics and Procedural Outcomes with Infarct Evolution
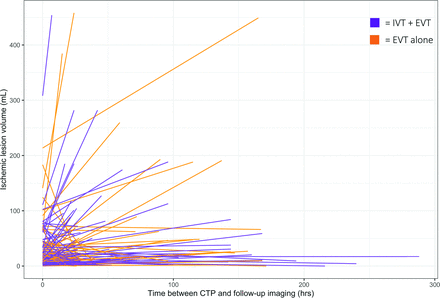
Univariable analyses showed that better collateral status was negatively associated with substantial infarct growth, and early re-occlusion of the target artery at 24-hour follow-up imaging was positively associated with substantial infarct growth. In addition, the number of attempts during EVT and the occurrence of any hemorrhage were positively associated with substantial infarct growth (Online Supplemental Data). Notably, reperfusion (eTICI) was not associated with infarct evolution. The distribution of infarct evolution stratified by reperfusion subgroup is shown in Fig 3.
Infarct evolution between baseline CTP and follow-up imaging on either DWI or NCCT for patients who received IVT and EVT (purple) and patients who underwent EVT alone (orange). A, The left panel shows data for all included patients.
After adjustment for confounders, better collateral status and a more distal occlusion location were negatively associated with substantial infarct growth. The number of attempts during EVT and the occurrence of any hemorrhage were positively associated with substantial infarct growth. Early re-occlusion of the target artery was not associated with substantial infarct growth in multivariable analysis. For all included variables, the variance inflation factors were <1.5, indicating no correlation between the included independent variables (Online Supplemental Data). An exploratory analysis in a subgroup of patients without any hemorrhagic transformation (n = 103) consistently showed that better collateral status and more distal occlusion location were negatively associated with substantial infarct growth.
Infarct Evolution for Patients Who Received IVT and EVT versus EVT Alone
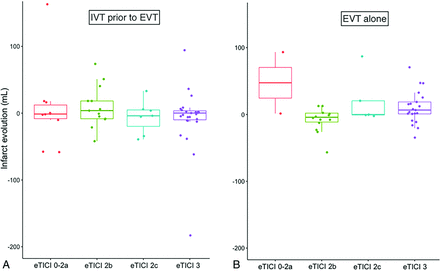
Substantial infarct growth (ie, infarct growth of >10 mL) occurred in 27/81 (33%) patients with IVT and EVT versus 27/64 (42%) patients who underwent EVT alone (P = .3). After adjustment for confounders, substantial infarct growth was not significantly associated with the administration of IVT and EVT (adjusted OR = 0.63; 95% CI, 0.30–1.32; P = .2). Boxplots showing the infarct growth per subgroup are provided in Fig 4.
Boxplots showing infarct evolution (milliliters) for patients who received IVT and EVT (A) and patients who underwent EVT alone (B), with eTICI 0-2a versus eTICI 2b versus eTICI 2c versus eTICI3 reperfusion.
Infarct Evolution for Patients with and without Successful Reperfusion
One hundred twelve (84%) patients achieved successful reperfusion after EVT. Patients with successful reperfusion showed lower median infarct evolution rates compared with patients without successful reperfusion (1 [IQR = 7–20] mL versus 15 [IQR = 2–71] mL), though this difference was not statistically significant (P = .2). From 59 patients with complete reperfusion (ie, eTICI 3), 20 (34%) showed substantial infarct growth.
Effect of Follow-up CTA or MRA Recanalization Status on Infarct Evolution
Follow-up CTA or MRA was available for 132 patients and showed incomplete patency of the target artery in 10% of patients receiving IVT and EVT versus in 15% of patients receiving EVT alone. However, this difference was not statistically significant (P = .3). In multivariable analysis, early re-occlusion of the target artery, assessed on follow-up CTA or MRA, was not associated with infarct growth (adjusted OR = 1.48; 95% CI, 0.28–7.83).
DISCUSSION
In this post hoc analysis of the MR CLEAN-NO IV trial, we did not observe a statistically significant difference in infarct evolution between directly admitted patients who received IVT and EVT versus patients who underwent EVT alone within 4.5 hours after symptom onset. Overall, successful reperfusion rates were similar in patients who received IVT and EVT versus EVT alone. Furthermore, our results demonstrated that collateral status, occlusion location, the number of attempts during EVT, and occurrence of any hemorrhage were statistically significantly associated with substantial infarct growth in patients who received IVT and EVT or EVT alone within 4.5 hours after symptom onset.
Our results showed that re-occlusion on follow-up imaging was not uncommon. However, frequencies of re-occlusion were comparable between both groups. Most interesting, re-occlusion on follow-up imaging was not statistically significantly associated with substantial infarct growth after adjusting for potential confounders. However, this nonintuitive finding might be explained by the fact that our sample size was limited and, therefore, potentially underpowered to detect a clear association. The observed rates of re-occlusion on follow-up imaging are in line with a previous study assessing vessel patency at 24-hour follow-up imaging using the mAOL score.34 Other studies assessing re-occlusion after EVT reported rates of early re-occlusion ranging from 3% to 9%. However, these studies used different imaging techniques and grading systems to assess the vessel patency on follow-up imaging (eg, 24-hour follow-up angiography using the Qureshi grading scheme).35,36
Our results showed that substantial infarct growth was associated with the number of attempts during EVT, which is in line with a previous large prospective study from multiple stroke registries.20 In addition, our results suggested that in the hyperacute (0–4.5 hour) time window, patients with poor collaterals have a higher likelihood of substantial infarct growth compared with patients with good collaterals. This finding is also in concordance with previous research in patients with stroke who underwent EVT within 6 hours of symptom onset.19,21
If replicated, the relatively high frequency of re-occlusion within 24 hours after endovascular treatment could imply that there might be a potential added benefit of thrombolytic therapy in addition to EVT to improve functional outcome after stroke. This possibility would also be in line with the preliminary findings from the Chemical Optimization of Cerebral Embolectomy (CHOICE) trial, which showed that adjunct intra-arterial alteplase in patients with large-vessel occlusion stroke resulted in a greater likelihood of excellent neurologic outcome at 90 days.37 Also, the authors showed that additional intra-arterial thrombolysis was associated with an increased likelihood of achieving excellent angiographic reperfusion (ie, eTICI 2c–3). However, the proportion of patients with infarct growth between baseline and follow-up imaging was not statistically significantly different between both study groups. This result could imply that additional factors such as, for example, microvascular perfusion may also contribute to functional outcome at 90 days and that these factors might be affected by additional thrombolytic therapy in patients treated with EVT.
Several limitations to our study should be noted. First, selection bias may have been introduced because CTP was not mandatory for inclusion in the MR CLEAN-NO IV trial and CTP was performed according to local imaging protocols. Of note, not all centers routinely performed CTP in every admitted patient with suspected stroke. A total of 228 (41%) patients in the MR CLEAN-NO IV had CTP available from 17 participating centers. Of these 228 patients, 145 (64%) patients had baseline CTP with follow-up NCCT or MR imaging available, leading to a relatively small sample size. However, the baseline, imaging, and outcome characteristics of patients without follow-up imaging were comparable with those in the overall MR CLEAN-NO IV population. Second, the MR CLEAN-NO IV trial had no standardized CTP acquisition protocol, and CTP data were acquired according to local acquisition protocols per site, possibly introducing differences in CTP ischemic core volume estimations.38 However, all CTP data were centrally processed using a previously described single postprocessing protocol.27
Furthermore, differences in CTP results that are caused by differences in acquisition protocols are commonly largely driven by differences in contrast medium injection protocols,38 and because the particular contrast medium injection protocols from centers in the MR CLEAN-NO IV were similar, we expect that the effect of using data from different acquisition protocols is limited. Third, FIV was measured on both 24-hour and 1-week follow-up NCCT and MR imaging. This practice could have affected the accuracy of our FIV assessments because it is known that edema affects the FIV on NCCT after stroke, and it can be challenging to distinguish edema from infarcted tissue on NCCT.39 However, the FIVs were not different for patients who received a median 24-hour follow-up DWI versus patients with 24-hour follow-up NCCT. In addition, it has been demonstrated that FIV assessed on 24-hour NCCT is equally strongly associated with functional outcome as the FIV measured on 1-week NCCT, regardless of the fact that infarct growth between 24-hour and 1-week imaging is common.24
Fourth, hemorrhagic regions were included in the final infarct lesion, possibly affecting our results. An exploratory analysis in a subgroup of patients without any hemorrhagic transformation (n = 103) consistently showed that collateral status and occlusion location were associated with substantial infarct growth. Excluding all patients with hemorrhagic transformation from our analyses could potentially introduce bias because it is not well-known how infarct growth changes with time and what the tempo of blood-brain barrier disruption and development of hemorrhagic transformation is.40
It is known that CTP may overestimate the FIV (ie, the “ghost infarct core concept”), especially in patients with successful reperfusion in the early time window.41 However, rates of overestimation of >10 mL were comparable with rates previously reported in a post hoc analysis of the Highly Effective Reperfusion evaluated in Multiple Endovascular Stroke (HERMES) trials collaboration.42 Similarly, we found that CTP ischemic core overestimation by syngo.via predominantly occurred in the white matter. Because previous studies have shown that ischemic core thresholds might differ between gray and white matter,43 future studies focusing on improving white matter ischemic core estimation by syngo.via should consider this difference.
Finally, the timing of follow-up scans had a wide range (1–288 hours posttreatment). Because we showed that infarct growth was common in our population, the timing of follow-up imaging could have affected the accuracy of FIV measurements. A pooled analysis on this topic from all trials investigating the noninferiority of EVT alone is warranted for confirmation of whether infarct growth differs between patients who received IVT and EVT versus patients who underwent EVT alone. Ideally, follow-up imaging should be acquired at similar time points using a single technique.
CONCLUSIONS
No statistically significant difference in infarct evolution was found in patients who received IVT and EVT versus patients who underwent EVT alone. Collateral status, occlusion location, and number of attempts during EVT are significantly associated with substantial infarct growth in IVT-eligible patients who undergo EVT.
Footnotes
B.J. Emmer and C.B.L.M. Majoie are shared last authors.
The MR CLEAN-NO IV trial was part of the Collaboration for New Treatments of Acute Stroke (CONTRAST) consortium. The CONTRAST consortium acknowledges the support from the Netherlands Cardiovascular Research Initiative, an initiative of the Dutch Heart Foundation (CVON2015-01: CONTRAST) and from the Brain Foundation Netherlands (HA2015.01.06). The collaboration project is additionally financed by the Ministry of Economic Affairs by means of the public-private partnerships allowance made available by Top Sector Life Sciences & Health to stimulate public-private partnerships (LSHM17016). This work was funded, in part, through unrestricted funding by Stryker, Medtronic, and Cerenovus.
The funding sources were not involved in study design, monitoring, data collection, statistical analyses, interpretation of results, or manuscript writing.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received October 3, 2022.
- Accepted after revision January 31, 2023.
- © 2023 by American Journal of Neuroradiology