Abstract
SUMMARY: Patients with coronavirus disease 2019 (COVID-19) may have symptoms of anosmia or partial loss of the sense of smell, often accompanied by changes in taste. We report 5 cases (3 with anosmia) of adult patients with COVID-19 in whom injury to the olfactory bulbs was interpreted as microbleeding or abnormal enhancement on MR imaging. The patients had persistent headache (n = 4) or motor deficits (n = 1). This olfactory bulb injury may be the mechanism by which the Severe Acute Respiratory Syndrome coronavirus 2 causes olfactory dysfunction.
ABBREVIATIONS:
- COVID-19
- coronavirus disease 2019
- SARS-CoV-2
- Severe Acute Respiratory Syndrome coronavirus 2
Coronavirus has the human respiratory system as its main target but also has neuroinvasive capabilities and can spread from the respiratory tract to the CNS.1-3 Therefore, patients with coronavirus disease 19 (COVID-19) may present with neurologic symptomatology with repercussions on imaging examinations,4⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-18 and these have been described in association with ischemic infarct,8,9 hemorrhage,11 acute hemorrhagic necrotizing encephalopathy,10 cerebral venous thrombosis,13 and diffuse leukoencephalopathy with microhemorrhage.15
Transmission from person to person occurs mainly by direct contact or droplets spread by coughing or sneezing by an infected individual with Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2).5,19 Symptoms of COVID-19 usually appear after an incubation period of about 5 days. The most common symptoms are fever, cough, fatigue, headache, and dyspnea.5,19,20 In the most severe cases, patients may develop pneumonia, acute respiratory failure, distress syndrome, and acute heart problems.5,19,20
Anosmia or partial loss of the sense of smell, usually accompanied by changes in taste, is a frequent symptom that helps in the diagnosis of COVID-19.21-28 It is often a transitory phenomenon, lasting just a few weeks.21 However, the mechanism by which anosmia occurs has not yet been established.29
The hypothesis is that the virus enters the central nervous system through the first neurons of the olfactory pathway, also called olfactory sensory neurons, located in the olfactory mucosa. The olfactory mucosa is a specialized neuroepithelium located in the highest portion of the nasal cavity in direct contact with the external environment below to the cribriform plate.1 Therefore, the virus crosses the cribriform plate to reach the olfactory bulbs, which contain the second olfactory neurons.1,30
There are currently only 2 reports evaluating olfactory bulb imaging, and they are discordant.18,31 The first report showed bilateral inflammatory obstruction of the olfactory clefts that was confirmed on MR imaging of the nasal cavity, but no anomalies of the olfactory bulbs and tracts.31 The second study reported a case with anosmia evaluated with 3D-CISS T2WI, which demonstrated severe enlargement and an abnormal high signal intensity on T2, being interpreted as bilateral olfactory bulb edema and also olfactory cleft mild edema.18 The control MR imaging (D24) showed a reduction in the volume of the bulbs.18
To our knowledge, no other report has evaluated the characteristics of the olfactory bulb, especially using fat-suppressed T1WI. Also, no report evaluates and shows the presence of bleeding or a break in the blood-brain barrier in the olfactory bulbs and tracts as the possible pathophysiology of olfactory neuropathy associated with COVID-19.
Thus, in this study, the authors demonstrate by MR imaging that a possible mechanism by which the SARS-CoV-2 causes olfactory dysfunction is by affecting, intracranially, the olfactory bulbs by a likely microvascular phenomenon.
MATERIALS AND METHODS
This retrospective study was approved by the institutional review board of the ethics committee of Universidade Federal de Pernambuco, Brazil. Informed consent was waived.
All scans were initially analyzed by the institution’s own neuroradiologists. Subsequently, all images were reviewed independently by 2 neuroradiologists (M.F.V.V.A. and O.Q.C.F, who were certified by the Ministry of Education and Culture of Brazil and the Brazilian College of Radiology) with 30 and 18 years, respectively, of neuroradiology experience, with no discordant results. MR imaging was indicated mainly because of a persistent incapacitating headache.
The intensity of the olfactory bulbs is defined as normal when the bulbs have the same cortical intensity, as typically seen in healthy controls. Abnormal olfactory bulb intensity is defined when the bulb is more hyperintense than the cortex on T1WI and STIR.
After gadolinium injection on T1WI, enhancement of the olfactory bulbs is defined when they become more hyperintense in comparison with their intensity on pregadolinium T1WI. However, when there is only the postgadolinium T1WI and the bulb is more hyperintense than the normal cortex, these features represent olfactory bulb intensity abnormality and may be an enhancement or microbleeding (methemoglobin), as interpreted in the present study. Microbleeding (methemoglobin) in the olfactory bulb is considered when there is a hyperintense olfactory bulb, compared with the normal cortex or the normal contralateral bulb, on pregadolinium fat-suppressed T1WI.
Brain MR imaging of patients with COVID-19 was evaluated from April 1, 2020, to May 18, 2020. Five patients were included in this study because their brain MRIs assessed their olfactory bulbs appropriately, with at least 2 sequences with thin slices examining the anterior cranial fossa. All 5 patients were evaluated with 2 coronal sequences with thin slices: postcontrast fat-suppressed T1WI and STIR. Only 1 patient also had thin-slice pregadolinium fat-suppressed T1WI.
The brain MRIs of the patients were performed on two 1.5T machines with the main technical parameters of the sequences described as follows. The coronal fat-suppressed T1WI (spectral presaturation with inversion recovery) parameters were, respectively, on both MR imaging machines the following: TR/TE = 561–605/15–9 ms, matrix = 256–88, FOV = 190–150 mm, thickness = 3 mm, 3.5-mm section, coronal orientation, bandwidth = 181–96.6 Hz, time = 3.39–4.49 minutes, NEX = 1–3.
The coronal STIR sequence had the following parameters in each MR imaging machine, respectively: TR/TE = 4000–2650/51–90 ms, TI = 180 ms, matrix = 256–224, FOV = 190–150 mm, thickness = 3–3.45 mm, section orientation = coronal, bandwidth = 190–232.4 Hz, time = 2.46–3.42 minutes, and NEX = 1–2.
RESULTS
All 5 patients with COVID-19 (On-line Table) had fever, headache, and cough. The medical indications for the performance of MR imaging were persistent headache (n = 4) or motor deficit (n = 1). All 5 patients had injury to the olfactory bulbs demonstrated by MR imaging with the following sequences: coronal pre-contrast (Fig 1A) and post-contrast fat suppression T1WI (Figs 1B and 2A, -D) and coronal STIR (Fig 1C).
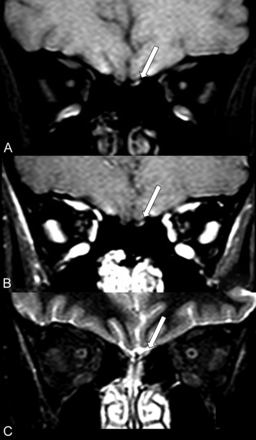
MR imaging shows probably microbleeding (methemoglobin) in the left olfactory bulb of a patient (case 1) with COVID-19 and anosmia. The left olfactory bulb (long arrows) has partial hyperintensity on precontrast fat-suppressed T1WI (A) and also on postcontrast fat-suppressed T1WI (B) and STIR (C).
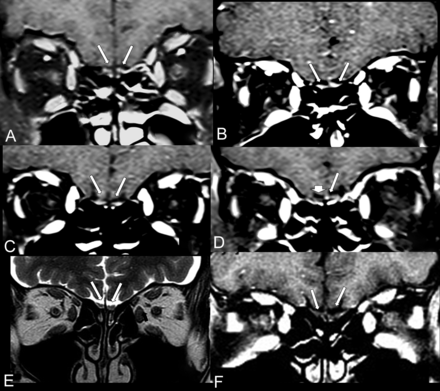
The coronal postcontrast fat-suppressed T1WI shows hyperintensity suggestive of enhancement or methemoglobin in the olfactory bulbs of 4 patients with COVID-19 (A–D; cases 2–5) compared with a healthy patient with normal olfactory bulbs (E and F). The coronal postcontrast fat-suppressed T1WI in 3 patients with COVID-19 (A–C; cases 2–4) shows that both olfactory bulbs (long arrows) are small oval images that are hyperintense with contrast, having signal intensity higher than the intensity of the cortex. D, A patient (case 5) with COVID-19 shows hyperintensity only on the left bulb (long arrow), the right olfactory bulb being normal (short arrow). In a healthy 60-year-old man, the coronal T2WI (E) and the postcontrast fat-suppressed T1WI (F) demonstrate normal olfactory bulbs (long arrows), which are isointense to the cortex and normally hypointense on postgadolinium sequence (F).
The only patient who had pregadolinium fat-suppressed T1WI (case 1) showed a small hyperintensity in the left olfactory bulb (Fig 1A), which remained hyperintense on the postgadolinium sequence (Fig 1B) and also on STIR (Fig 1C). This finding was suggestive of a small area of methemoglobin in the left olfactory bulb in this patient with anosmia.
In the 4 patients who did not have a pregadolinium sequence, we did not have information about anosmia in 1 patient (Fig 2A; case 2), and 2 of them with anosmia (Fig 2B, -C; Cases 3 and 4) showed hyperintensity suggestive of enhancement of both olfactory bulbs following gadolinium injection. However, only in the patient with COVID-19 without clinical anosmia (case 5) was there a suggestive enhancement in the left olfactory bulb (Fig 2D). The case 5 had a negative result of the CSF real-time RT-PCR for SARS-CoV-2. The differential diagnosis in these cases is mainly microbleeding (methemoglobin) because the pregadolinium sequence was not performed. Coronal STIR of the anterior cranial fossa did not show any abnormality in the olfactory bulbs in these 4 patients.
MR imaging of a healthy individual was used as a comparative control (Fig 2E, -F) to demonstrate that the normal olfactory bulbs do not enhance and are isointense to the cerebral cortex.
DISCUSSION
This case series demonstrates abnormal intensity of the olfactory bulbs in 5 adult patients with COVID-19, three of whom had anosmia. In 1 patient (case 1), the abnormal intensity could represent microbleeding (methemoglobin). However, in the other 4 patients, it could represent abnormal enhancement or microbleeding (methemoglobin) because they only underwent the sequence after injection of gadolinium in fat-suppressed T1WI.
Previously, it was demonstrated, using an experimental mouse model, that the SARS-CoV could travel from the nose to the olfactory bulb.32 Regarding the SAR-CoV infection, there was a time delay of about 60 hours from the time of nasal infection until the detection of the virus in the olfactory bulb.1,32
The literature has already reported that some other viruses can use the olfactory nerve as a shortcut into the CNS, such as influenza A virus, herpesviruses, poliovirus, paramyxoviruses, vesicular stomatitis virus, rabies virus, parainfluenza virus, adenoviruses, Japanese encephalitis virus, West Nile virus, chikungunya virus, La Crosse virus, mouse hepatitis virus, and bunya viruses.30 van Riel et al30 have reported that “Viral infection of the CNS can lead to damage from infection of nerve cells per se, from the immune response, or from a combination of both. Clinical consequences range from nervous dysfunction in the absence of histopathological changes to severe meningoencephalitis and neurodegenerative disease.” However, to our knowledge, no study evaluated and documented, by brain MR imaging, the abnormalities such as microbleeding and/or enhancement in the olfactory bulbs occurring in these other kinds of viruses.
Probably, the impairment of olfactory function is much more frequent in COVID-19 because, strictly speaking, unilateral anosmia can only be detected through a detailed physical examination. The patient hardly perceives unilateral anosmia.
Recognizing this hypersignal in the olfactory bulbs on the thin slices of pre- and/or postgadolinium fat-suppressed T1WI, identified in this study, may help to suggest or support the etiologic diagnosis of COVID-19 during and after this new pandemic.
Thus, we suggest, henceforth, including in the routine brain MR imaging protocol at least a sequence with coronal thin-slice pre- and/or postgadolinium fat-suppressed T1WI in the anterior fossa of the cranium. This feature will be more important in cases of refractory headache associated or not with other symptoms and signs such as fever and anosmia.
The weakness of this work is that it is a retrospective study with only a few cases in which it was possible to evaluate the olfactory bulbs. Brain MR imaging of patients with COVID-19 has not been routinely scheduled to adequately evaluate the olfactory bulbs because other neurologic complications were being investigated. The distortion at the air-tissue interface in fat-suppressed T1WI makes the findings somewhat difficult to interpret, but it seems that the images are true abnormal lesions along the olfactory bulbs. Future prospective studies geared to evaluating the olfactory bulbs with a larger sample size will be needed to confirm our findings.
In conclusion, the authors demonstrated by MR imaging that a possible mechanism by which the SARS-CoV-2 causes olfactory dysfunction is by affecting, intracranially, the olfactory bulbs, with development of a microvascular phenomenon and injury such as microbleeding and/or a blood-brain barrier break. This seems to be the first time that a neuroimaging study has documented this type of olfactory bulb injury in patients with COVID-19.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received May 20, 2020.
- Accepted after revision June 1, 2020.
- © 2020 by American Journal of Neuroradiology