Abstract
BACKGROUND AND PURPOSE: Ultrasound is generally considered to have a minor role in guiding biopsies for deep head and neck space lesions. However, the ultrasound fusion technique may have the potential to change this opinion. This study evaluated the feasibility, histopathologic yield, and safety of ultrasound fusion–guided core needle biopsies for deep head and neck space lesions.
MATERIALS AND METHODS: From March 2021 to April 2022, patients with primary deep head and neck space lesions were prospectively included in this study. Ultrasound fusion was performed with contemporaneous CT, MR imaging, or PET/CT studies, and ultrasound fusion–guided core needle biopsy was performed by using a Micro-Convex probe via 4 different needle approaches. Feasibility, histopathologic results, and biopsy-related complications were observed. Descriptive statistics were applied.
RESULTS: Ultrasound-guided biopsy was feasible in all 16 patients (11 women and 5 men; mean age 46 [SD, 16] years; range, 16–76 years). The lesions were located in the parapharyngeal space, infratemporal fossa, and skull base, with a median diameter of 3.8 cm (range, 2.2–6.5 cm). An adequate and definite histopathologic yield was obtained in 15/16 (93.8%) patients; among them, 4/15 lesions (26.7%) were malignant, and 11/15 (73.6%) were benign. No major complications occurred. Minor complications were noted in 2 of the 16 (12.5%) patients (self-limiting inflammation in 1 and bleeding in 1).
CONCLUSIONS: This study demonstrates that ultrasound fusion–guided biopsy of deep head and neck space lesions is feasible and safe, with a high histopathologic yield.
ABBREVIATIONS:
- H&N
- head and neck
- US
- ultrasound
Lesions arising from deep head and neck (H&N) spaces are uncommon, but clinical management is challenging due to anatomic complexity and histologic diversity.1,2 Preoperative biopsy remains standard for better counseling and treatment planning. Open biopsy is used but should be carefully chosen due to risks, while percutaneous imaging-guided biopsy is now increasingly performed. CT guidance is a well-established biopsy method for H&N lesions,3,4 but reports involving deep H&N spaces are few and mostly limited to small samples of patients.5⇓-7 In addition, infrared navigation–guided biopsy has been explored in a small number of patients at a few institutes.8⇓-10 MR imaging guidance is not widely used because of the longer acquisition times, higher cost, and the need for an open-configuration MR imaging system and MR imaging–compatible needles.3,11
Ultrasound (US) guidance, with its inherent advantages of real-time imaging capability, rapid performance time, vessel depiction without intravenous contrast material, flexibility and portability, lack of radiation exposure, and high cost-effectiveness, is generally the first-line choice for biopsies on many H&N masses. However, to date, almost all researchers consider US unsuitable for the deep H&N region,3,5⇓⇓⇓-9,12 for 2 reasons: First, acoustic degradation increases with depth, especially when the depth is >4 cm,12 increasing the difficulty in delineating a target reliably; and second, osseous or air structures preclude an adequate acoustic window for US scanning.
US fusion has been widely used in biopsy and ablation procedures of the liver, kidney, and prostate.13,14 However, it has scarcely been applied in deep H&N spaces, such as the parapharyngeal space, infratemporal fossa, and skull base. To solve the above 2 issues, we have combined 2 advances; first, we have used US fusion imaging with CT, MR imaging, or PET/CT. Second, instead of a traditional Linear Array Probe, we have used a Micro-Convex probe (PVT-382BT; 3.0-5.0 MHz; Canon Medical Systems) for deeper acoustic penetration and better suitability for the narrow space between the bony structures. Thus, our purpose was to evaluate the feasibility, pathologic diagnostic value, and safety of US fusion–guided biopsies for deep H&N space lesions.
MATERIALS AND METHODS
Patients
This study was approved by the institutional review board. Each patient was informed of the risks and consented to the procedure. From March 1, 2021, to April 30, 2022, sixteen patients with primary deep H&N space lesions were prospectively enrolled in this study. The decision regarding biopsies was discussed by a multidisciplinary team, including otolaryngologists, oncologists, and radiologists. The indications for US fusion–guided biopsy were the following: 1) deep H&N space lesions that were difficult to identify on routine B-mode US, and 2) lesions that needed to selectively target the solid, enhancing components or hypermetabolic tissue. The contraindications were as follows: 1) pure cystic lesions; 2) patients who had a pacemaker and could not be in the magnetic field; 3) patients who could not maintain a fixed position during the procedure; and 4) patients with a bleeding disorder. For lesions with contiguous spread into adjacent anatomic spaces, the epicenter of the mass was used to classify the location.
US Fusion Process
The details of CT/MR imaging and whole-body PET/CT techniques are described in the Online Supplemental Data. All data were collected within 1 week before the biopsy, stored in DICOM format, and imported into the fusion platform (Smart-Fusion; Canon Medical Systems) packaged with the US system (Aplio i900; Canon Medical Systems). An embedded electromagnetic navigation system (3D Guidance; Northern Digital) was used for probe tracking.
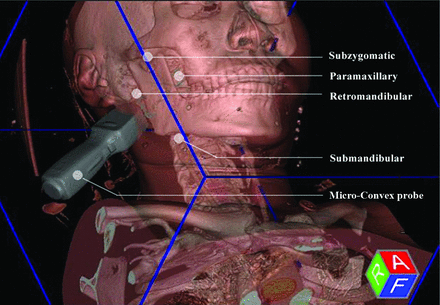
One of 2 radiologists with >5 or 10 years of experience performed the procedure. Patients maintained a stable supine position, tilting the head away from the side of the target lesion if necessary. The fusion process was broadly similar to the previous description of abdominal organs, which is based on the same principles of electromagnetic tracking and registration of the cross-sectional images with the live US image.13,14 Particularly for the deep H&N space lesions in this study, we used a Micro-Convex probe, which had a smaller volume, deeper penetration, and better suitability to scan via the narrow acoustic window compared with the traditional Linear Array Probe. The planned US scanning and biopsy approaches included the subzygomatic, retromandibular, paramaxillary, and submandibular approaches (Fig 1). In addition, we used the plane of the globe for initial registration, followed by manual adjustment of the position by point-to-point registration and confirmation by color Doppler flow imaging and the overlay mode. The common anatomic points included optic nerves, carotid bifurcations, nasal tip, hyoid bone, and the thyroid. The registration errors were ≤5 mm and validated by >4 different points in the overlay mode. When registration was successful, the cross-sectional images were coordinated and moved simultaneously with real-time US scanning. The operator could then confidently locate the biopsy target (Online Video). This process took approximately 2–5 minutes for an experienced operator.
Schematic of US fusion–guided biopsy, with the use of a Micro-Convex probe and 4 available needle approaches.
US Fusion–Guided Biopsy
We performed all biopsies with the patient under local anesthesia with 1% lidocaine and used an 18-ga semiautomated side-cutting biopsy needle with a slot of 2.2 cm (Magnum; C.R. Bard). Guided by real-time US fusion imaging, the operator inserted the needle through a planned approach to obtain ≥2 core specimens, either by the freehand technique or assisted by the needle holder. Meanwhile, the blood vessels and necrotic areas of the lesions were avoided.
Patients were clinically monitored for at least 30 minutes after the biopsy, and they were followed up between 1–3 days and 1 month after the procedure to document any additional short-term or delayed complications.
Statistical Analysis
Statistical analysis was performed using SPSS, Version 22.0 (IBM). Continuous data are described as medians and interquartile ranges or mean (SD), according to the Shapiro-Wilk normality test.
RESULTS
Patient Characteristics and Procedural Details
In the 14-month period, 16 patients (11 women and 5 men; mean age, 46 [SD, 16] years; range, 16–76 years) underwent US fusion–guided biopsy of deep H&N space lesions. Presenting symptoms varied, though the 2 most common were swelling (7/16, 43.8%) and tinnitus (4/16, 25.0%). The long-axis diameter of the lesions ranged from 2.2 to 6.5 cm (median, 3.8 cm; interquartile range, 2.7–4.3 cm). The center of the lesion was most frequently the parapharyngeal space (9/16, 56.3%), followed by the infratemporal fossa (4/16, 25%); 3 lesions (3/16, 18.8%) were located in the skull base. CT (11/16, 68.8%) was the most commonly used technique for US fusion, followed by MR imaging (4/16, 25.0%) and PET/CT (1/16, 6.3%). The retromandibular approach (7/16, 43.8%) was the most used needle approach, followed by the submandibular (4/16, 25.0%), subzygomatic (3/16, 18.8%), and paramaxillary approaches (2/16, 12.5%). The number of needle passes ranged from 2 to 3 (median, 2.5). Detailed information on the patient characteristics and procedural details are provided in the Online Supplemental Data.
Histopathologic Yield
The US fusion–guided biopsies were technically successful in all patients. Adequate samples were obtained for definite pathologic diagnosis in 15 of the 16 biopsies, and the diagnostic rate was 93.8%. Among those, 26.7% (4/15) were malignant, and the remaining 73.6% (11/15) were benign. Schwannoma (Fig 2), meningioma (Fig 3), and lymphatic malformations (Fig 4 and Online Video) were the top 3 benign pathologic types; others included solitary fibrous tumor (1/15, 6.7%), Warthin tumor (1/15, 6.7%), pleomorphic adenoma (1/15, 6.7%), and paraganglioma (1/15, 6.7%). The malignant diagnoses included invasive meningioma (1/15, 6.7%), acinic cell carcinoma of the parotid gland (1/15, 6.7%), squamous cell carcinoma (1/15, 6.7%), and nasopharyngeal carcinoma (1/15, 6.7%) (Fig 5). One biopsy (1/16, 6.3%) was nondiagnostic due to inadequate tissue sampling.
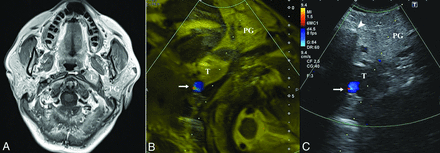
A 58-year-old woman without clinical presentation (patient No. 2). A, Axial T1-weighted postcontrast MR image shows a left parapharyngeal lesion with heterogeneous hyperintensity and a demarcated border. The target lesion is located deep in the parotid gland, and the ICA (arrow) is displaced medially. After a successful fusion of MR imaging (B) and US (C), MR imaging facilitates the accurate localization of the target lesion, which is ill-defined on US, with the ICA (arrow) confirmed on the color Doppler mode and the overlay mode (yellow mask). The dotted line indicates the expected needle path via the retromandibular approach. The histopathologic yield was schwannoma. T indicates target lesion; PG, parotid gland.
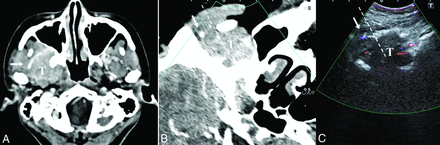
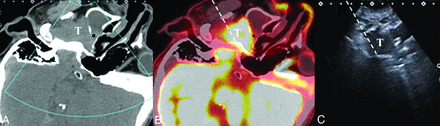
A 30-year-old woman with maxillofacial swelling, pain and dizziness for 2 years (patient No. 3). A, The axial contrast-enhanced CT image shows a homogeneously enhancing lesion involving the infratemporal fossa and pterygopalatine fossa. A branch of the maxillary artery runs superficially around the lesion (arrow). After a successful fusion of CT (B) and US (C), we can locate the target lesion that is occult on US due to the acoustic shadow of the mandible. Color Doppler US helps to avoid the maxillary artery (arrow). The dashed line indicates the expected needle path via the subzygomatic approach. Histopathology demonstrated meningioma (meningothelial subtype, World Health Organization grade I). T indicates target lesion.
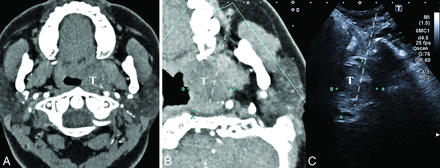
A 32-year-old woman with aural fullness, tinnitus, and hearing loss for 1 year (patient No. 10). A, The axial contrast-enhanced CT image shows a parapharyngeal lesion at the level of the alveolar ridge with mild and heterogeneous enhancement, constricting the auditory tube. After successful fusion with CT (B), a heterogeneous low-echoic lesion can be identified and located on US. C, The paramaxillary approach is chosen (dashed line). Histopathology demonstrated lymphatic malformation. T indicates target lesion.
A 39-year-old man with headache for 1 month (patient No. 14). A multiplanar image of US–PET/CT fusion with the patient’s head tilted to the contralateral side. A, The axial plain CT shows an ill-defined lesion centered in the infratemporal fossa with bony destruction. B, The axial fused [18F] FDG-PET/CT reveals a high focal uptake. C, The target lesion is occult behind the acoustic shadow of the coronoid process on US, but a selective biopsy can be guided under fusion images. The subzygomatic approach is chosen (dashed line). Histopathology revealed nasopharyngeal carcinoma. T indicates target lesion.
Biopsy-Related Complications
No major complications occurred after the biopsies. Minor complications were observed 1 day after the biopsy in 2 of 16 (12.5%) patients, both of whom were diagnosed with lymphatic malformation: One had mild inflammation of the puncture site and was treated conservatively in the outpatient clinic, and the other had self-limited bleeding that presented with blood-tinged sputum and required no intervention.
Clinical Management
Four patients (4/16, 25%) with diagnostic biopsies underwent subsequent surgical excision, and a concordant pathologic diagnosis was obtained in 100% (4/4) of them. One patient (1/16, 6.3%) diagnosed with lymphatic malformation underwent sclerotherapy by tansoral injection of pingyangmycin. One patient (1/16, 6.3%) diagnosed with nasopharyngeal carcinoma was treated by radiation therapy and chemotherapy; the original surgical plan was canceled. One patient (1/16, 6.3%) diagnosed with squamous cell carcinoma refused further treatments. The remainder with benign or less invasive lesions (8/16, 50%) underwent clinical and imaging follow-up, following multidisciplinary discussion and consensus. In our 1 patient with a nondiagnostic specimen, the subsequent surgical pathology revealed low-grade myoepithelial carcinoma ex pleomorphic adenoma.
DISCUSSION
This pilot study showed that US fusion–guided biopsy was feasible, effective, and safe, with a high histopathologic yield for deep H&N space lesions. To our knowledge, this is the first report of the application of US fusion to this challenging area and may play a role in the work-up of lesions involving the parapharyngeal space, infratemporal fossa, and skull base.
US-guided biopsy is an ideal first-line technique for cervicofacial masses, such as the thyroid, superficial lobe of the parotid gland, and cervical lymph nodes. US has played a minor role in the deep H&N spaces overlaid by bony structures such as the maxilla, mandible, mastoid, and styloid process, as well as the air-containing aerodigestive tract. This role is understandable when operators used only a large Linear Array Probe for scanning these areas, with obstructing anatomy overlying them. As reported in a recent study, preoperative needle biopsies were not feasible in 35% (42/120) of patients with parapharyngeal tumors because ultrasound or transoral palpation could not localize the lesions.15 However, our study has demonstrated the feasibility of US-guided biopsy for deep H&N spaces when combined with imaging fusion techniques and modification of probe and needle-path selection. In our biopsy procedure, US fusion provided resolution compensation, while the Micro-Convex probe accommodated the acoustic window and reduced the blind zone. All the deep H&N space lesions in this study were technically accessible, even a small skull base lesion measuring approximately 2 cm.
CT guidance has been considered a valuable tool in the biopsy of deep H&N lesions. However, conventional CT guidance lacks real-time feedback during the biopsy procedure, so the operator must confirm the needle path by intermittent scans. Real-time CT-fluoroscopy guidance or conebeam CT guidance is less widely used and can increase the radiation exposure to operators.16,17 In comparison, US fusion–guided biopsy is a real-time procedure, crucial for avoiding blood vessels or other structures without radiation exposure. Previous studies have shown a diagnostic rate of CT-guided core needle biopsy of 73%–96.8% in lesions involving the H&N as a whole.4⇓⇓-7 The average size of all target lesions was approximately 2.4–3.3 cm,4⇓-6 including superficial and lower cervical masses. For emerging infrared navigation-guided biopsy, the diagnostic rate was approximately 90%.8⇓-10 The target lesion size was reported in only 1 study of 8 patients, ranging from 2.5 to 9.0 cm (median, 3.7 cm).10 The diagnostic rate in our study was 93.8% (15/16), with a median lesion size of 3.8 cm (range, 2.2–6.5 cm), which is similar to other guidance modalities.
One biopsy sample of a parapharyngeal lesion measuring 3.8 cm was inadequate in this study, yielding a nondiagnostic biopsy rate of 6.3% (1/16). A small lesion size and necrotic components may contribute to nondiagnostic specimens.7,11 In retrospectively reviewing this unsuccessful biopsy, we postulated that patient movement during needle insertion may have affected the registration accuracy. The operator should take note of the patient’s position and re-perform registration if necessary.
US fusion–guided biopsy was demonstrated to be safe without major complications in this study, in accordance with the results of other studies. Major complications after biopsy of deep H&N masses are rare. The risk of cranial nerve injury remains a theoretic concern but has not been demonstrated in the literature to date. Two cases of severe vascular complications after CT-guided biopsy of a H&N lesion have been reported.18,19 According to the authors, avoidance of vascular structures was critical, especially for patients with prior neck surgery and radiation therapy. The minor complication rate was 12.5% (2/16), including mild inflammation of the puncture site and self-limited blood-tinged sputum. Mild infection and bleeding were the most reported minor complications after image-guided biopsy in the H&N.3⇓⇓⇓⇓⇓⇓⇓-11 Other minor complications included vasovagal reaction, transient facial palsy, and CSF leakage, which did not occur in this study.
The major limitation of our study was the small sample size. Because the incidence of deep H&N space lesions is low, US fusion–guided biopsy must be further evaluated in more patients and in multiple centers. In addition, we could not always compare histopathologic concordance between the biopsy and surgical specimens because most of the lesions in this study were benign, and the choice of surgery was avoided or postponed.
CONCLUSIONS
This pilot study demonstrates the feasibility, efficacy, and safety of US-guided percutaneous core needle biopsy for lesions in deep H&N spaces. Our findings may broaden the clinical application of the US fusion technique and contribute to diagnosing deep H&N space lesions accurately and conveniently.
Footnotes
Xiaoju Li and Jian Li contributed equally to this article.
This work was supported by the National Natural Science Foundation of China (Grant No.92059201).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received July 14, 2022.
- Accepted after revision December 31, 2022.
- © 2023 by American Journal of Neuroradiology