Abstract
SUMMARY: Sinonasal and skull base tumors are a heterogeneous group of neoplasms with considerable histologic variation and overlapping imaging features. In 2022, the World Health Organization updated the head and neck tumor classification, further emphasizing the importance of molecular data and genetic alterations in sinonasal neoplasms. The changes include the addition of new entities and discussion of emerging entities, as well as changes to the taxonomy and characterization of tumors. The new classification focuses on entities that develop in these sites either exclusively (eg, olfactory neuroblastoma) or most frequently. Another change includes reduction in the number of categories by creating separate category-specific chapters for soft-tissue, hematolymphoid, and neuroectodermal lesions. In this review, we briefly discuss the various categories in the new classification with a more detailed description of the 2 new entities (SWItch/Sucrose Non-Fermentable complex–deficient sinonasal carcinomas and human papillomavirus–related multiphenotypic sinonasal carcinoma). We also highlight the emerging entities including IDH–mutant sinonasal malignancies and DEK-AFF2 carcinoma, presently classified as sinonasal undifferentiated carcinoma and nonkeratinizing squamous cell carcinoma, respectively.
ABBREVIATIONS:
- HPV
- human papillomavirus
- NUT
- nuclear protein in testis
- REAH
- respiratory epithelial adenomatoid hamartoma
- SCCa
- squamous cell carcinoma
- SMARCB1
- SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1
- SNT
- sinonasal tract
- SNUC
- sinonasal undifferentiated carcinomas
- SWI/SNF
- SWItch/Sucrose Non-Fermentable
- WHO
- World Health Organization
The 5th edition of the World Health Organization (WHO) classification of head and neck tumors was released online in 2022 with a focus on distinctive molecular and genetic characteristics of tumors including in the sinonasal tract (SNT).1 The increasing incorporation of molecular and genetic information reflects our continuously evolving understanding of the underlying biologic underpinnings and is broadly true for most updated WHO tumor classifications in other specialties. Apart from increasing the diagnostic accuracy, these genetic alterations also have prognostic and predictive value, allowing a more personalized approach. The 5th edition of the WHO Classification of Head and Neck Tumors has several changes from the 4th edition (2017), which consider the multidimensional nature of tumor classification with a better consensus definition and provide greater insight into the pathogenesis of these tumors.1,2
The SNT (including the nasal cavity, paranasal sinuses, and the skull base) is an anatomic region that has high morphologic, phenotypic, and genotypic diversity of neoplasms with considerable overlap of histologic features. The new classification focusses on either entities that develop in these sites exclusively (eg, olfactory neuroblastoma) or those with a preponderance of disease in this location. Another major change was exclusion of tumor subcategories that do not occur exclusively or predominantly in the SNT region, to avoid redundancy and duplication. These were moved to their own dedicated chapters, such as soft-tissue tumors, melanocytic tumors, neuroendocrine neoplasms (such as paraganglioma), and so forth, with few exceptions.3 Adamantinomatous craniopharyngioma is the only entity that has been moved to the SNT (from the nasopharynx in the 4th edition), because the SNT is the exclusive ectopic location for these tumors. A new chapter with genetic tumor syndromes was added with 15 entities with predominant head and neck manifestations. The Table outlines the changes to the major categories of SNTs. The new classification allows a more logical and stratified approach, with successive entities showing progression from benign to malignant and higher-grade tumors. There is a new category for mesenchymal tumors of the SNT and a category for “other” sinonasal tumors that includes olfactory neuroblastoma.1⇓-3 Imaging characterization of these new entities is still at an early stage with significant overlap in the radiographic presentation. Nevertheless, it is important for the radiologist to be aware of the clinical course of these tumors, including the epidemiologic features and how they respond to treatment. A great example would be the DEK-AFF2 subtype of nonkeratinizing sqaumous cell carcinoma (SCCa), which classically occurs along the posterior part of middle turbinate, with excellent response to immunotherapy.
Summary of changes including deletion, addition, and reclassification of SNT tumors in the 5th edition of the WHO Classification 2022
Molecular and Genetic Markers
Histopathologic analysis remains a vital part of the pathologic work-up of SNTs, despite the challenging morphologic overlap. Molecular testing including immunohistochemistry has now become routine for pathologic evaluation of the SNTs in developed countries. Genetic profiling has caused an explosion in the subclassification of sinonasal malignancies, particularly focused on subtyping for improved prognostication and treatment, though profiling is still lagging for head and neck tumors, compared with other systems like CNS tumors. Although genetic panels for head and neck tumors are still at an early stage, aberrations are now widely reported across different tumor types with varying degrees of sensitivity. A case in point would be the identification of IDH 1/2 mutation in 50–80% of tumors currently classified as sinonasal undifferentiated carcinoma (SNUC), and a smaller proportion of cases classified as large cell neuroendocrine carcinoma or olfactory neuroblastoma. IDH 1/2 mutation in these tumors is associated with less aggressive clinical behavior and is now noted to be a distinct emerging entity (Fig 1).4,5 Similarly, identification of the DICER mutation in nasal chondromesenchymal hamartoma would signify an underlying tumor-predisposition disorder with recognition of several seemingly unrelated neoplasms, including pulmonary blastoma, multinodular goiter, and thyroid carcinomas (Fig 2).1,6 Newly recognized entities like SWItch/Sucrose Non-Fermentable (SWI/SNF) complex–deficient sinonasal carcinoma (provisionally included as SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 [SMARCB1]–deficient sinonasal carcinoma in the 4th edition) also rely on a lack of SMARCB1/INI1 protein expression on immunohistochemistry. The 5th edition adds and expands on the genetic arrangements included in the definition of multiple entities, including that of sinonasal papillomas and hamartomas. The Online Supplemental Data outline the common molecular and genetic mutations in the newly recognized sinonasal tumors along with their pathogenetic pathway. The histologic grading of SNTs is an important independent predictor of tumor behavior. However, the propensity for grade transformation, progression, and recurrence is also dependent on the molecular subtypes, underlining their importance not just for the surgeons and oncologists but also for radiologists. It is almost certain that as the field evolves, additional neoplastic entities will be identified in the near future.
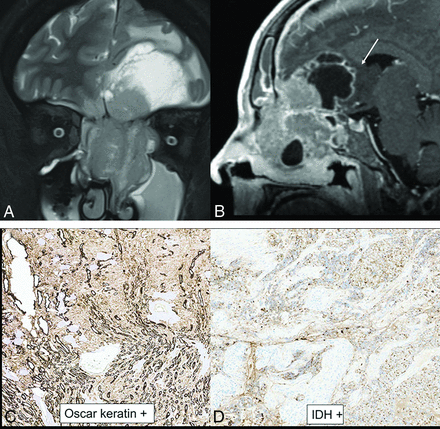
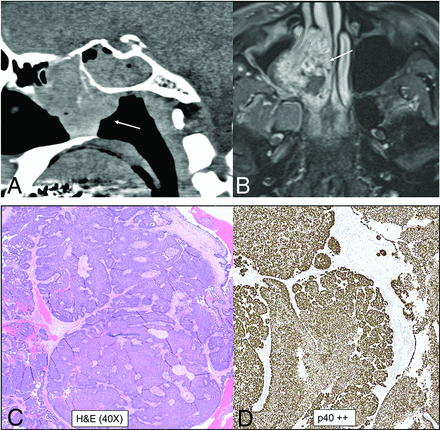
IDH2-mutated SNUC. A large, infiltrative sinonasal mass with homogeneous, mild T2 hyperintensity (A), avid contrast enhancement, and erosion of the skull base with intracranial extension (B). A large intratumoral cyst (B, arrow) with enhancement of the cyst walls. Radiographic differentials included SNUC and olfactory neuroblastoma. Histopathology shows sheet-like and nested growth of cells with hyperchromatic nuclei, with diffuse positivity on cytokeratin (Oscar) stain (C) distinguishing carcinomas (like SNUC) from nonepithelial malignancies. Immunohistochemistry for IDH1/2 (D) shows strong and diffuse granular cytoplasmic staining, confirming an IDH-mutation.
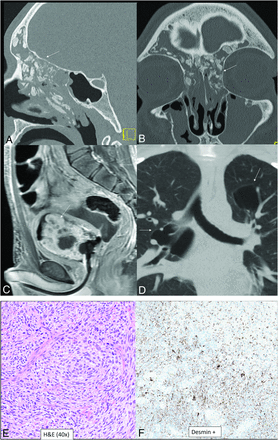
DICER1-mutant tumors. Nasal chondromesenchymal hamartomas with pathogenic germline variation in DICER1 presenting with extensive soft-tissue “masses” in the frontal and ethmoid sinuses involving the anterior skull base with calcific/ossific bodies within the matrix (A and B, arrows). Embryonal bladder rhabdomyosarcoma in the same patient, which is also one of the hallmark tumors associated with a DICER mutation. Bladder rhabdomyosarcoma is seen as a heterogeneous, solid, avidly-enhancing mass within the bladder lumen (C, arrow). Multiple thin-walled pulmonary cysts (D, arrow) with tiny septal nodules are indeterminate but may represent type Ir (regressed) pleuropulmonary blastomas in the setting of the DICER1 mutation. The histopathology of a bladder lesion reveals embryonal rhabdomyosarcoma with diffuse anaplasia (E) and extensive cartilaginous differentiation and diffusely positive staining for desmin (muscle marker) (F).
Hamartomas
Sinonasal hamartomas include respiratory epithelial adenomatoid hamartoma (REAH), seromucinous hamartoma, and nasal chondromesenchymal hamartoma. REAH is a benign glandular neoplasm of the sinonasal cavities, which presents in isolation or secondary to allergy and inflammatory processes such as sinonasal polyps. This is the most common hamartoma of the SNT, seen in old adults with a strong male predilection, classically located along the posterior septum and olfactory cells. It has also been described in association with inverted papilloma and low-grade sinonasal adenocarcinoma, with reports suggesting that it might be the precursor for the latter. Imaging reveals exophytic polypoid homogeneous lesions with smooth expansion of the olfactory cleft without bony erosion, though identification can be challenging when associated with sinonasal polyposis. Seromucinous hamartomas are polypoid masses seen typically in the posterior nasal septum and nasopharynx, ranging from a few millimeters to a few centimeters. On imaging, these lesions cannot be differentiated from REAH, with both exhibiting a characteristic crescentic “half-moon” appearance on the sagittal images when situated along the olfactory cleft (Fig 3). The differential diagnosis for seromucinous hamartomas and REAH includes inflammatory polyp, encephalocele, olfactory neuroblastoma, and low-grade nonintestinal type adenocarcinoma.7⇓-9
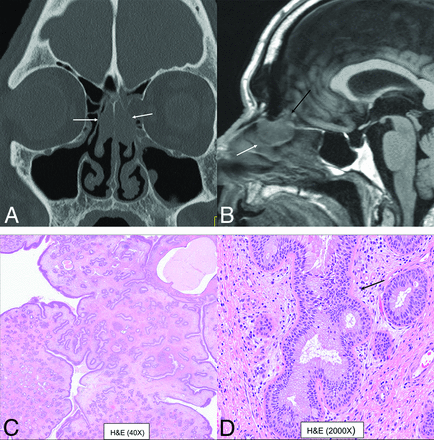
Bilateral REAH. Smooth polypoid lesions noted within the nasal cavity bilaterally (A and B, white arrows). The lesion expands the olfactory cleft (B, black arrow) with smooth remodeling, without erosive changes. This cannot be differentiated from seromucinous hamartoma with both exhibiting a crescentic (half-moon) shape on sagittal images (B). Histopathology revealed prominent glandular structures of varying sizes (C). Glands are lined with ciliated respiratory epithelium with bland nuclei and mucinous gland metaplasia (D, black arrow).
Chondromesenchymal hamartoma is the most distinctive lesion in this category, with a strong association with the DICER1 (tumor-suppressor gene) mutation, usually presenting in infants. This is most common within the ethmoid sinuses and is frequently (25%) bilateral, with imaging revealing a complex, solid, and cystic heterogeneous appearance with calcification and bony erosions (Fig 2). DICER1 syndrome is a highly pleiotropic tumor-predisposition entity that has been increasingly recognized during the past decade. It is no longer limited to pulmonary blastoma, with other associations including multinodular goiter, thyroid carcinoma, ovarian sex cord stromal tumors, and pituitary blastoma. The most common symptoms for all these hamartomatous lesions include nasal obstruction, rhinorrhea, and epistaxis.8,10,11
Respiratory Epithelial Lesions
Sinonasal Papillomas.
Sinonasal papillomas, also known as Schneiderian papillomas, are benign sinonasal neoplasms that arise from the Schneiderian epithelium of the nasal cavity and paranasal sinuses. They have 3 different histologic subtypes, including inverted papilloma (most common, 50%–80%), exophytic papilloma (20%–50%), and oncocytic papilloma (least common, 5%).12 These account for 0.5%–4% of all nasal tumors and are usually seen in the fourth-to-fifth decades of life. There have been important updates to the etiology and pathogenesis of these lesions with somatic epidermal growth factor receptor (EGFR) mutations reported in 90% of inverted papillomas and 80% of carcinomas developing from these lesions. Inverted papillomas generally occur along the lateral wall of the nasal cavity and the paranasal sinuses (most commonly the ethmoid or maxillary sinus). Imaging shows a soft-tissue mass centered along the middle meatus with frequent calcification and a classic convoluted cerebriform pattern, seen on both T2-weighted and contrast-enhanced T1-weighted images. These, however, cannot be distinguished from sinonasal carcinomas and are treated by en bloc resection, given the high risk of malignant transformation. Oncocytic papillomas also involve the lateral wall of the nasal cavity and appear similar to inverted sinonasal papilloma on imaging but have a lower risk of malignant transformation. They, however, may show T1-shortening (Fig 4) with multiple mucinous cysts along with lack of focal osteitis, all of which may help differentiate them from inverted papillomas.13,14 Exophytic sinonasal papilloma is a benign SNT epithelial neoplasm usually arising from the lower anterior nasal septum with a broad base. It is around 2 cm but lacks any specific imaging appearance. They are invariably unilateral and can involve the lateral nasal walls generally without involvement of the paranasal sinuses.15,16
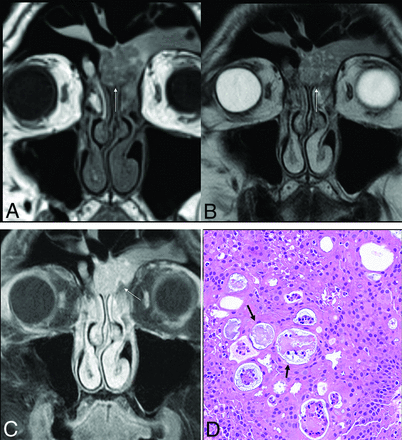
Sinonasal papilloma, oncocytic type, in the left frontal sinus in a 78-year-old man. Smooth polypoid lesions are noted within the nasal cavity with intrinsic T1 hyperintensity (A, arrow). The lesions obstruct the frontoethmoidal recess with trapped proteinaceous content in the frontal sinus. Lesions show a “cerebriform” pattern on the T2-weighted image (B, arrow) with avid enhancement (C, arrow). Histopathology reveals characteristic oncocytic cuboidal-to-columnar cells and intraepithelial microcysts with mucin and/or neutrophilic microabscesses (D, black arrows).
Carcinomas.
Carcinomas of the SNT account for 3% of malignant head and neck neoplasms. Two new entities have been added to the category of sinonasal carcinomas and include human papillomavirus (HPV)-related multiphenotypic sinonasal carcinoma (provisionally included as HPV-related carcinoma with adenoid cystic-like features in the 4th edition) and SWI/SNF complex–deficient sinonasal carcinoma (provisionally included as SMARCB1-deficient sinonasal carcinoma in the 4th edition).1 Neuroendocrine carcinoma has been removed from this section in the current edition and is discussed separately. Other carcinomas have been retained from the prior edition and include SCCa (keratinizing and nonkeratinizing), NUT carcinoma, and lymphoepithelial and undifferentiated carcinoma. Although these lesions have different anatomic preferences, imaging findings are overall similar, with aggressive features, presenting as a poorly circumscribed soft-tissue mass with irregular margins often with bone destruction and frequent multifocal and metastatic disease.17 Keratinizing SCCa is rarely associated with HPV, may be associated with sinonasal papillomas, and is histologically identical to any other affected site. This is the most common malignancy of the nasal vestibule, with the maxillary antrum with the lateral nasal wall being the other common locations. Nonkeratinizing SCCa is a distinctive sinonasal tumor that most commonly arises in the nasal cavity or maxillary sinuses. It is characterized by minimal-to-no keratinization and is strongly positive for immunohistochemical markers such as p40 and CK5/6, which also help in differentiating them from morphologic mimickers like NUT carcinoma, SNUC, and neuroendocrine tumors.18,19
NUT carcinoma was a new entity in the previous (4th) edition and is better defined in the current 5th edition.1 It was previously called NUT midline carcinoma due to proclivity for midline sites. NUT carcinoma has been recognized in up to 18% of upper aerodigestive tract poorly differentiated carcinomas, with recognition aided by improved testing techniques and increased availability of commercial antibodies. The defining genetic feature of NUT carcinomas includes fusion of the NUTM1 gene, most commonly to BRD4.20 There are <100 cases described in the literature, limiting detailed description of imaging features. Limited series and published case reports have described aggressive imaging findings, like those in the mediastinum, with bony hyperostosis, internal mineralization, avid contrast enhancement, and high FDG uptake (Fig 5).8,21 Other tumors in this category include sinonasal lymphoepithelial carcinoma, teratocarcinosarcoma, and SNUC. However, with advancement in molecular markers and identification of newly defined entities (eg, SWI/SNF complex–deficient sinonasal carcinomas), the diagnosis of poorly differentiated or undifferentiated entities like SNUC is becoming less common.1
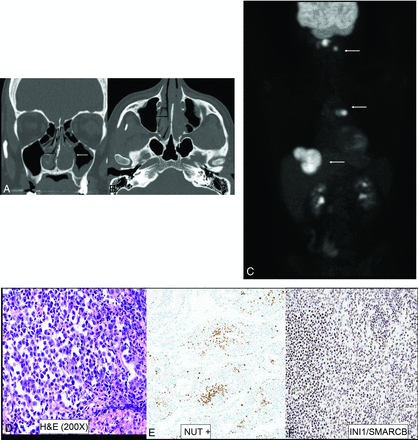
NUT sinonasal carcinoma in a 22-year-old man. Left nasal cavity mass (A and B, white arrows) with hyperostosis of the septum (black arrows). Metastatic disease with lesions in the lymph nodes, liver, and lung (C, white arrows). H&E stain (D) shows sheets and nests of high-grade tumor cells with zones of tumor necrosis. The tumor exhibits positive staining on NUT immunohistochemistry (E) with preserved INI1 staining (F) (ruling out SMARCB1 deficiency). Genetic analysis revealed NUT-BRD4 fusion, the defining feature of NUT carcinomas.
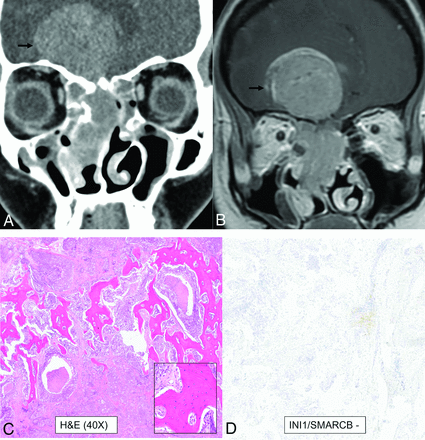
SWI/SNF complex–deficient sinonasal carcinomas were provisionally included as SMARCB1-deficient sinonasal carcinoma in the 4th edition. These are poorly differentiated, highly-aggressive, and infiltrative epithelial malignancies defined by loss of 1 SWI/SNF complex subunit (most commonly SMARCB1 or less frequently SMARCA4), usually presenting at an advanced stage.1 These comprise 1%–3% of sinonasal carcinomas and 3%–20% of tumors diagnosed as SNUC. These tumors are seen in young and old adults (age range, 33–78 years) with no sex predilection. The SMARCB1-deficient carcinomas involve the paranasal sinuses (most commonly the ethmoid), whereas the SMARCA4-deficient carcinoma predominantly involves the nasal cavity. These are destructive lesions with common involvement of the skull base and orbits, frequent (50%) calcification, a “hair-on-end” pattern periosteal reaction, and increased uptake on PET. On MR imaging, these are variably hyperintense on T2WI and show avid enhancement with low ADC values. Given the recent identification of this tumor, detailed studies on the imaging pattern are limited. The differentiation is based on immunohistochemical markers with complete loss of expression of SMARCB1 (INI1-stain negative) or SMARCB4 (BRG1-stain negative) in the SWI/SNF complex–deficient sinonasal carcinomas (Fig 6).21,22 The common differential for an aggressive sinonasal tumor along the anterior skull base thus includes SWI/SNF complex–deficient sinonasal carcinomas, SNUC, neuroendocrine tumor, and olfactory neuroblastomas.
SWI/SNF complex–deficient sinonasal carcinoma in a 30-year-old woman. A large infiltrative sinonasal mass with intracranial extension (A and B, black arrows) and marked peritumoral edema in the frontal lobes. Peritumoral cysts were noted in the intracranial component. Radiologic differentials included olfactory neuroblastoma, SNUC, SWI/SNF complex–deficient (SMARCB-deficient) tumor, NUT carcinoma, and high-grade neuroendocrine tumor. H&E stain shows a basaloid low-power appearance (C) with hyaline-appearing cytoplasm showing a “pink” cell appearance, in which the tumor cells are somewhat plasmacytoid (C, inset). Complete loss of INI1/SMARCB1 (D) expression by immunohistochemistry defines this tumor, as seen here. Immunostaining for NUT and synaptophysin (a neuroendocrine marker) were negative.
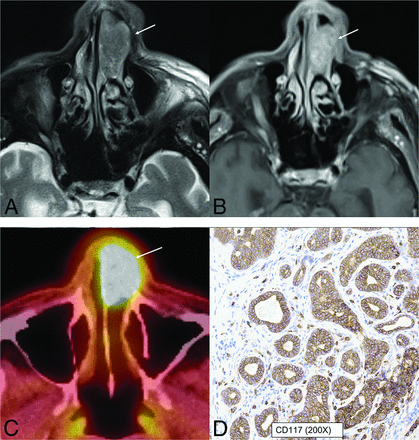
HPV-associated multiphenotypic sinonasal carcinoma is the other new tumor in the 5th edition that was provisionally included as HPV-related carcinoma with adenoid cystic-like features in the 4th edition.1,2 This unique neoplasm exhibits histologic features of both surface-derived and salivary gland carcinomas, hence the name “multiphenotypic.” This is associated with high-risk HPV (usually type 33) and, unlike adenoid cystic carcinoma, is restricted to the SNT. Most tumors affect the nasal cavity (89%), with a predilection for the turbinate, with or without involvement of the paranasal sinuses. There is a wide age range of presentation from the second-to-ninth decades, with slightly higher predilection for women (1.5:1). Tumors show myoepithelial differentiation on histology, with strong and diffuse nuclear and cytoplasmic p16 immunoreactivity (a surrogate marker for HPV) and do not have the MYB/MYBL1 fusions that characterize most adenoid cystic carcinomas.22,23 Descriptions of the imaging appearance are limited and include nasal cavity origin, infiltrative appearance with heterogenous T2 hyperintensity, periosteal reaction, and hyperostotic changes (Fig 7). Despite having adenoid cystic features, perineural tumor spread is uncommon. No cystic nodal metastases have yet been described, despite the HPV origin of these tumors.8,24 This is different from HPV-induced SCCa, which originates along the tonsillar crypts, is most commonly caused by type 16 HPV, has nonkeratinizing SCCa morphology, and in which regional/nodal metastases are common.
HPV-associated multiphenotypic sinonasal carcinoma in an 85-year-old man. A well-circumscribed, low T2-signal mass (A, arrow) with avid contrast enhancement (B, arrow) seen within the left nasal cavity with smooth expansion and high FDG uptake on PET/CT (C, arrow). CD117 (c-kit) (D) highlights the tumor ducts in a staining pattern that is the inverse of the myoepithelial cell markers. Findings of in situ hybridization for HPV (E6/E7) RNA was positive.
Adenocarcinomas.
Adenocarcinomas of the SNT arise from the respiratory epithelium or the underlying seromucinous glands. These malignancies were previously (3rd edition) divided into salivary type and nonsalivary type. The salivary type (eg, adenoid cystic carcinomas) since then has been removed from the SNT chapter and placed under the salivary gland chapter. The nonsalivary type is further divided into intestinal-type and nonintestinal-type adenocarcinomas. Intestinal-type adenocarcinoma is the second most common type of sinonasal adenocarcinoma, most often localized in the ethmoid sinus (40%), followed by the nasal cavity (25%). These tumors are aggressive and frequently involve adjacent structures, including the orbit, pterygopalatine fossa, and infratemporal fossa, along with intracranial extension. Nonintestinal-type adenocarcinomas are usually low-grade, often arising in the nasal cavity (along nasal turbinates) with an imaging appearance similar to that of inverted papillomas. The low-grade subtypes present as solid masses, filling the nasal cavity and/or paranasal sinuses, with no osseous destruction. Emerging molecular studies suggest that these low-grade adenocarcinomas have distinctive mutations (eg, CTNNB1) or fusions (eg, ETV6-NTRK3). Imaging shows a poorly circumscribed, enhancing SNT mass and is used to assess the disease burden and local extension.25,26
Mesenchymal Tumors and Other Tumors of the SNT
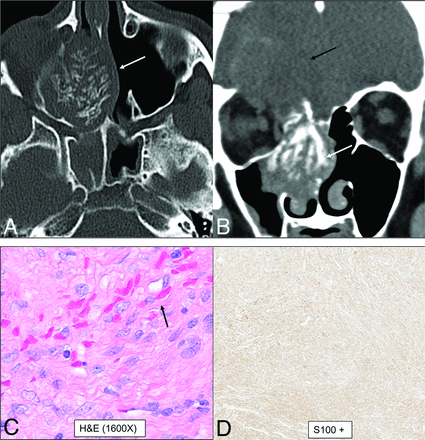
Multiple categories of tumor have been separately described in the new edition, including benign soft-tissue tumors and borderline, low-grade malignant soft-tissue tumors. However, some soft-tissue tumors that occur exclusively or predominantly in the sinonasal region were retained in the newly created category of mesenchymal tumors. These include angiofibroma, glomangiopericytoma, biphenotypic sinonasal sarcoma, and chordoma. Among these, biphenotypic sinonasal sarcoma is the relatively recent entity, introduced only in the 4th edition (2017).1,2 These tumors have neural and myogenic features and are histologically similar to cellular schwannomas or malignant peripheral nerve sheath tumors. Rearrangement of the PAX3 gene is required for the diagnosis of these tumors. They are characteristically seen in middle-aged women, arising from the nasal cavity or ethmoid sinus. Imaging reveals locally aggressive nasoethmoid-enhancing masses that can erode through the orbits and skull base with frequent hyperostotic bony changes (Fig 8).
Biphenotypic sinonasal sarcoma (low-grade sinonasal sarcoma with neural and myogenic features) in a 68-year-old woman. Marked intralesional hyperostotic changes are noted within the nasal component of the tumor (A and B, white arrows). The tumor is well-marginated but locally aggressive with intracranial extension (B, black arrows). H&E stain (C) shows the tumor growing as fascicles of uniform spindle cells with minimal mitotic activity or atypia and overt rhabdomyoblastic differentiation in the form of strap cells (C, arrow). Diffusely positive staining of S-100 (nerve sheath tumor marker) seen as diffuse brown staining (D). Fluorescence in site hybridization testing was positive for PAX3 gene rearrangement, supporting the above diagnosis.
Neuroectodermal tumor and the hematolymphoid tumor categories have also been removed and described in dedicated chapters. The only exception is olfactory neuroblastoma, which has been retained in the “other” category of tumors because these tumors occur predominantly within the SNT. These are malignant, neuroectodermal neoplasms with a bimodal age distribution and are confined to the cribriform plate, superior turbinate, and superior half of the nasal septum. With intracranial extension, peritumoral cysts between the mass and underlying brain are often present. Tumor cysts and speckled calcifications are characteristic of the intacranial component of the tumor, though not exclusive. These tumors show diffuse staining for conventional neuroendocrine markers and S-100 protein, with high avidity on radionucleotide studies including indium-111 (111In) pentetreotide (OctreoScan; https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020314s012lbl.pdf) and gallium-67 (67Ga) DOTATATE PET/CT studies.27,28
Adamantinomatous craniopharyngioma is also a new entity in the “other” category. This is, however, only for taxonomic clarity, with the entity being shifted from the nasopharynx (4th edition) to the SNT. Adamantinomatous craniopharyngioma arises from the cellular elements related to the Rathke pouch and can occur anywhere along the craniopharyngeal canal, most commonly in the suprasellar region and very rarely in the nasopharynx and SNT. Although the latter is exceedingly rare, the SNTs are nevertheless the only ectopic location of this tumor. These are seen as cystic masses with peripheral enhancement and prominent calcifications.1,29,30
Emerging Entities
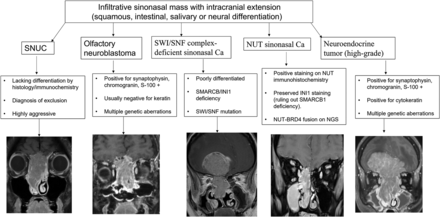
Advancements in genetic studies have led to better understanding and more accurate classification of undifferentiated carcinomas. Studies have shown that a significant proportion of what remains in the SNUC group has underlying IDH2 hotspot mutations with better prognosis. IDH2-mutated sinonasal carcinoma will likely be a separate tumor entity in future editions. The diagnosis of IDH2-mutated SNT is increasingly being made, given the availability of inexpensive immunohistochemical studies, and it is now recognized as the most common variant present in SNUC (accounting for 50%–88% of cases). The median age of patients with IDH2-mutated sinonasal carcinomas is 57 years, compared with 71 years in IDH2 wild-type, with better prognosis. These range from 2.5 to 7 cm, most commonly occurring in the nasal cavity and ethmoid sinus and usually present with advanced disease. Histologically, these mimic undifferentiated or poorly differentiated carcinomas but show IDH2-positivity (Fig 1).4,31 The radiographic differential for an aggressive sinonasal mass with orbital and intracranial extension would include SNUC (including the IDH-mutant subset) neuroendocrinal tumors, neuroectodermal tumors (like olfactory neuroblastoma), and tumors with defined molecular alterations (NUT, SMARCB1, and SMARCA4/A2), with significant overlap in imaging features (Fig 9).
Radiographic differentials of an invasive sinonasal mass with intracranial extension are broad with significant overlap in imaging features, including previously described characteristic findings like intratumoral cyst in olfactory neuroblastoma. The final diagnosis is now based primarily on immunohistochemistry and genetics rather than histology. The prognosis of these tumors is also widely variable depending on the underlying molecular and genetic expression. Ca indicates carcinoma.
Along similar lines, DEK-AFF2 carcinoma is an emerging entity, currently included as a subtype of nonkeratinizing SCCa, most commonly occurring within the nasal cavity with a peculiar propensity for the posterior part of the middle turbinate (Fig 10). Epidemiologic data on this entity are limited, with the largest series of 13 patients (Rooper et al32) having a median age at presentation of 56 years (range, 18–79 years ). Limited published data on this entity have shown that despite bland histologic features, these carcinomas are clinically aggressive tumors, with a higher risk of recurrence, nodal spread, and metastasis compared with the parent category. DEK-AFF2 carcinomas should be considered in the differential diagnosis of high-grade sinonasal malignancies. This subcategory, however, shows an excellent response to immunotherapy. There is increasing evidence that nonkeratinizing SCCa with DEK-AFF2 is a distinctive tumor entity and serves as a good example of how “poorly differentiated” and “undifferentiated” tumors are being continuously refined.32,33 RNA sequencing is increasingly being performed on multiple sinonasal neoplasms because immunohistochemical assays are not available, especially for newer entities. Finally, there is a newly created section dedicated to genetic tumor syndromes involving the head and neck. The initiative was undertaken to facilitate better understanding of the tumors and associated syndromes and to establish recommendations for monitoring and treating these patients. Many tumors that were thought to be sporadic earlier are now known to be a part of syndrome complex, with identification of specific mutations. A great example of this would be the PTEN germline mutation found in about 85% of patients with Cowden syndrome, characterized by multiple hamartomas involving the oral cavity.1,34
DEK-AFF2 carcinoma. A smooth homogeneous density mass (A, arrow) noted within the posterior ethmoid and nasal cavity with heterogeneous enhancement on MRI (B, arrow). The mass blocks the sphenoethmoidal recess with trapped secretions in the sphenoid sinus. The carcinoma demonstrates classic features of nonkeratinizing SCCa, with blunted papillae and invasion into the stroma as interconnecting ribbons on the H&E stains (C) with infiltrate of inflammatory cells, including lymphocytes and neutrophils. The tumor is diffusely positive for the squamous immunohistochemical marker p40 (D). To evaluate fusion-driven tumor, we performed targeted RNA sequencing, and DEK-AFF2 fusion was found, with breakpoints of DEK (exon 7) and AFF2 (exon 6).
CONCLUSIONS
In the past decade, molecular markers and genetics have revolutionized the taxonomy of sinonasal and skull base tumors, leading to recognition of new entities and more accurate understanding of the underlying pathogenesis. The most striking example of this change would be the SNUC tumors in which multiple new entities have been described on the basis of the underlying mutation. Radiologists should be abreast of these changes because discussion about these molecular markers and genetic changes is common during multidisciplinary tumor board meetings. This field is evolving, with continuous improvement in the classification system, recognition of new entities, and standardization in diagnosis, paving the way for targeted treatments.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received May 26, 2023.
- Accepted after revision June 22, 2023.
- © 2023 by American Journal of Neuroradiology