Abstract
BACKGROUND AND PURPOSE: Patients exhibiting acute dizziness or vertigo often represent a diagnostic challenge, and many undergo neuroimaging for stroke detection. We aimed to demonstrate the imaging outcomes of first-line emergency MR imaging among patients with acute dizziness or vertigo and to determine the clinical risk factors for stroke and other acute pathology.
MATERIALS AND METHODS: This retrospective study included consecutive patients with acute dizziness or vertigo referred for emergency MR imaging in a tertiary hospital during 5 years. We recorded and analyzed patient characteristics, relevant clinical information, and imaging outcomes. Risk score models were derived to predict which patients were more likely to present with positive MR imaging findings.
RESULTS: A total of 1169 patients were included. Acute stroke was found in 17%; other clinically significant pathology, in 8% of patients. In 75% of the patients, emergency MR imaging showed no significant abnormalities. Risk factors for acute stroke included older age, male sex, and a prevalence of cardiovascular risk factors and neurologic signs. Isolated dizziness had no discriminative power on imaging outcomes, and 14% of these patients showed acute stroke. Risk scores had only moderate performance in predicting acute ischemic stroke (receiver operating characteristic area under curve = 0.75) or any significant pathology (receiver operating characteristic area under curve = 0.70).
CONCLUSIONS: Acute dizziness and vertigo remain challenging even when emergency MR imaging is readily available. One in 4 patients had acute pathology on MR imaging. Predictors for acute pathology (older age, male sex, cardiovascular risk factors, and neurologic signs) may aid in patient selection for MR imaging, optimizing the yield and clinical impact of emergency neuroimaging. Low diagnostic yields of CT and internal acoustic canal MR imaging may offer an opportunity to reduce health care expenditures in the future.
ABBREVIATIONS:
- AIS
- acute ischemic stroke
- AUC
- area under the curve
- HINTS
- head impulse, nystagmus, and test of skew
- IQR
- interquartile range
- NPV
- negative predictive value
- NS
- nonsignificant findings
- PPV
- positive predictive value
- ROC
- receiver operating characteristic
- S
- significant-but-nonischemic pathology
- STANDING
- SponTAneous Nystagmus, Direction, head Impulse test, standiNG
Dizziness (including vertigo) is a rather common symptom among the general population, affecting 15%–35% at some point in their lives.1 According to the International Classification of Vestibular Disorders, dizziness is defined as a sensation of disturbed or impaired spatial orientation without a false or distorted sense of motion, whereas vertigo is described as a sensation of self-motion when no self-motion is occurring or the sensation of distorted self-motion during an otherwise normal head movement.2 Patients and physicians often use these terms interchangeably, which may lead to imprecision and inconsistency in patient care and research.3,4 In this article, vertigo denotes a vestibular symptom encompassing false spinning sensations, though the exact definition varies among studies.
Patients exhibiting acute dizziness or vertigo often represent a diagnostic challenge. Despite many patients being diagnosed with a benign peripheral vestibular disorder, some patients may need neuroimaging to rule out central causes of dizziness. Stroke, particularly vertebrobasilar acute ischemic stroke, is the primary differential diagnosis among central causes and is diagnosed in approximately 3%–5% of all emergency visits for dizziness and vertigo.5,6 Several bedside examination patterns, such as head impulse, nystagmus, and test of skew (HINTS) and SponTAneous Nystagmus, Direction, head Impulse test, standiNG (STANDING), and clinical risk scores (such as TriAGe+ and ABCD2) have been developed for early stroke detection.7⇓⇓-10 Despite these attempts to focus on high-risk patients, generally 30% to 50% of acutely dizzy emergency patients undergo neuroimaging.6,8,11 According to the American College of Radiology guidelines on dizziness (American College of Radiology Appropriateness Criteria), MR imaging is usually an appropriate imaging method when dizziness is accompanied by neurologic deficits, or the HINTS examination findings are consistent with central vertigo.12
Imaging options include CT/CTA and MR imaging. CT is the most used method to exclude stroke due to widespread availability and fast scan times, but it has a low sensitivity for stroke of around 30% among patients with acute dizziness and vertigo.13 CT is especially challenging in the posterior fossa, where vertebrobasilar acute ischemic strokes occur. Although less commonly used, conventional MR imaging has a higher sensitivity of 80%.13 If one included thin-section DWI with a 3-mm section thickness, an even higher sensitivity of 95% for posterior circulation stroke may be reached.14 Recently, MR imaging was shown to demonstrate a higher rate of critical findings15 and improved cost-effectiveness16 compared with CTA in emergency patients with dizziness.
In the United States, recent annual spending for neuroimaging dizziness is as high as US $88 million, of which MR imaging accounted for 70%, though a head CT scan was the most used test across settings.17 In total, neuroimaging was applied >376,000 times per year within 6 months of the first presentation with dizziness to an emergency department or an outpatient clinic.
Only a few studies have been published on the yield of emergency MR imaging in dizziness and vertigo. In a study among 188 emergency patients with dizziness or vertigo who underwent MR imaging, around a 20% acute stroke rate and a 17% rate in other significant abnormalities were reported.18 In this study, risk factors for acute stroke were age older than 50 years, a high number of cardiovascular risk factors, a short duration of symptoms, and at least 1 neurologic sign.18 A higher proportion of stroke (33%) has been prospectively recorded among selected emergency patients with acute-onset vertigo who did not have a previous diagnosis of peripheral vertigo.19
The primary aim of this study was to assess the imaging outcomes of emergency MR imaging among patients with acute dizziness or vertigo and to characterize these patients in terms of demographics, history, and specific signs and symptoms. We also aimed to demonstrate factors related to significant imaging outcomes to aid clinical decision-making and improve the effective use of emergency neuroimaging.
MATERIALS AND METHODS
This retrospective cohort study was conducted at Turku University Hospital, an academic tertiary care referral center with an approximate patient catchment area of 480,000. During the study period, the emergency radiology department had an Ingenia 3T system (Philips Healthcare) dedicated to emergency imaging only.20,21
Permission for this study was obtained from the hospital district board, and patient consent was waived due to the retrospective nature of the study. Consecutive emergency MR imaging scans obtained between April 2014 and January 2019 were retrospectively identified from the PACS and radiologic information systems using standard MR imaging codes. The MR imaging protocols varied, but most included routine sequences such as T1WI and T2WI, FLAIR, DWI (axial), SWI, 3D TOF arterial angiography, high-resolution T2-weighted sequences of the internal acoustic canal and inner ear (selected patients), and contrast-enhanced T1WI (selected patients). Imaging data were cross-referenced with those from the electronic medical records.
To identify cases with dizziness and vertigo, we queried the referrals with the keywords “dizziness” and “vertigo.” The retrospective study design did not allow us to reliably separate central and peripheral vertigo. Postoperative patients, patients with a ventriculoperitoneal shunt, and patients with a recent head injury were excluded because they almost always undergo neuroimaging if presenting with dizziness and may have specific complications that are not well-generalizable. Patients of all age groups, whether emergency admissions or inpatients, were included as long as the aforementioned keywords were featured in the clinical indication for the emergency MR imaging request. All patients had emergency MR imaging as a part of their routine care, and the decision to refer the patient was made by the attending physician on clinical grounds.
From the referrals, we recorded the patients’ demographic characteristics, cardiovascular risk factors, neurologic signs, and other clinical symptoms. Missing information was then retrieved from the electronic medical records. Imaging findings were recorded from the MR imaging reports. On the basis of the emergency MR imaging findings, the patients were allocated to one of the 3 groups: those with acute ischemic stroke (AIS), those with other significant-but-nonischemic pathology (S), and those with nonsignificant findings (NS). Nonischemic MR imaging findings deemed likely to be causative and clinically significant incidental findings were included in the S category. A finding was considered clinically significant if it led to a change in management or to further examinations. Scans showing incidental findings, anatomic variations, lack of novel findings, or notable progression in chronic brain diseases were considered nonsignificant because they would be unlikely to account for the acute dizziness. The MR imaging reports were evaluated and then classified by 2 fellowship-trained neuroradiologists (J.H. and M.N.), first separately and then together to achieve consensus. We did not record interobserver agreement. A clinical neurologist was consulted when necessary. For the patients in the AIS category, results of the preceding CT studies were noted if available.
Results are typically expressed as percentages, medians, interquartile ranges (IQRs), and ORs with 95% CIs. The normality assumptions were evaluated both visually and using the Shapiro Wilk test. At the univariate level, we used the χ2 test to compare nominal data and the Mann–Whitney U and the Kruskal–Wallis H tests as nonparametric tests to compare continuous variables that were not normally distributed. Optimal cutoff points for continuous variables were determined using the Youden J statistic. All variables were also entered into binary (2 outcome classes) and multinomial (3 outcome classes) logistic regression models. Variables that were statistically significant predictors at the multivariate level were then included in the risk scores for predicting significant imaging outcomes. Risk score points were derived by rounding the OR (or 1/OR) of the included variables to the nearest integer. The points were summed to form a risk score for each patient. A sample calculation is available in the Online Supplemental Data. Receiver operating characteristic (ROC) and area under the curve (AUC) with sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were used to evaluate the diagnostic ability of our model. The ROC AUC provides an overall estimate of the model classification accuracy (proportion of correctly classified patients). The optimal cutoff points for the risk scores to maximize sensitivity and specificity were determined by the Youden J statistic.
The data were analyzed using JMP for Mac (Version 16.1 Pro; SAS Institute, 1989–2019). P values < .05 were considered statistically significant.
RESULTS
From 8772 unique emergency MR imaging scans, the initial search identified 1419 patients, of whom a total of 1169 patients met the inclusion criteria. The median age was 61 (IQR, 45–71) years, ranging from 6 to 90 years. A narrow majority were female (n = 646, 55%). Most patients had undergone their MR imaging study <24 hours after the referral (82%), and the rest had a median delay of 1 day (IQR = 1–2 days).
AIS was present in 197 (17%) of the MR imaging studies (Online Supplemental Data). Ninety-seven patients (8%) had other significant pathology (S), and for the rest of the patients (n = 875, 75%), the MR imaging scans remained NS. Of the 197 patients with AIS, 171 (87%) underwent a head CT scan before MR imaging, usually on the same or the previous day. Acute pathology was suggested in only 62 (36%) of these CT studies. The cerebellum was the most common infarct location among patients with AIS, involved in 39% of the patients. Other infarcts were found in the cerebrum (23%), pons (10%), medulla oblongata (5%), thalamus (5%), basal ganglia (2%), mesencephalon (2%), and for 15% of patients in multiple aforementioned locations. Nonischemic significant findings included tumors and infections, among other rare findings, such as neurosarcoidosis or central pontine myelinolysis (Table 1). The most common incidental findings among the NS group were white matter hyperintensities (Table 2).
Significant-but-nonischemic emergency MR imaging findingsa
Nonsignificant emergency MR imaging findingsa
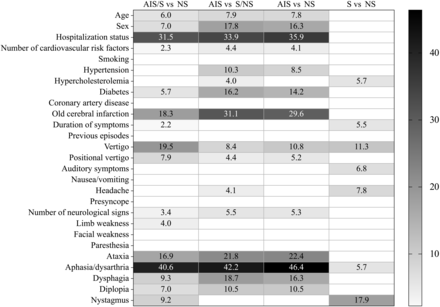
In the overall univariate analyses among the 3 findings groups (AIS/S/NS), numerous statistically significant associations were identified (Online Supplemental Data). Patients in the AIS group were more likely older, and men, and had a higher prevalence of cardiovascular risk factors and neurologic signs. A cutoff point for older age was 55 years. Patients in the S group had a high prevalence of headache and a long duration of symptoms. Patients with vertigo were less likely to present with any acute findings on MR imaging. Pair-wise group comparisons further elaborate these differences (Fig 1). Among the 126 patients with isolated dizziness, 14% had AIS, 10% had other significant findings, and 76% had nonsignificant findings, in similar proportions to patients with additional signs and symptoms (17% AIS, 8% S, 75% NS; P = .49).
Statistical test values between dichotomic imaging outcome groups. Only values with statistical significance (P < .05) are shown. The χ2 test was used for categoric variables, and the Mann-Whitney U test, for continuous variables.
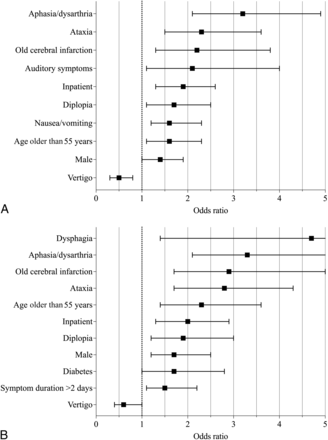
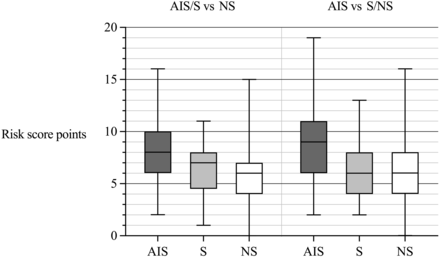
In a multivariate analysis, statistically significant predictors of clinically significant acute pathology (AIS/S) were aphasia/dysarthria, ataxia, old cerebral infarction, auditory symptoms, inpatient hospitalization status, diplopia, nausea/vomiting, age older than 55 years, male sex, and absence of vertigo (Fig 2). The risk score points for each variable are presented in the Online Supplemental Data. The ROC AUC for the risk score was 0.70. With a single cutoff of 6 points, the model had a sensitivity of 66% and a specificity of 64%. PPV was 38%, and NPV was 85%. The mean risk score was 7.6 points in the AIS/S group and 5.7 points in the NS group (Fig 3).
A, Variables predicting significant acute pathology (AIS/S versus NS) in emergency MR imaging with statistical significance (P < .05) in a multivariate analysis. B, Variables predicting acute ischemic stroke (AIS versus S/NS) in emergency MR imaging with statistical significance (P < .05) in a multivariate analysis.
Risk score point distributions for both scores within imaging outcome groups.
We also calculated a similar risk score for acute infarcts only (AIS versus S/NS) and found improved performance, with an ROC AUC of 0.75 (Fig 2). With a single cutoff of 8 points, sensitivity was 51%, specificity was 84%, PPV was 40%, and NPV was 90%. The mean risk score was 8.8 points in the AIS group and 5.6 points in the S/NS group (Fig 3).
To ensure that early MR imaging did not miss infarcts (false-negative), we determined whether patients with negative findings on DWI <48 hours after symptom onset had follow-up neuroimaging the following week. Among the 470 patients fulfilling these criteria, only 4 (0.9%) underwent follow-up CT or MR imaging. Only one (0.2%) of these patients, scanned because of new neurologic symptoms after vertigo had dissipated, had a small new cortical infarction on CT. All patients in the catchment area with a clinically meaningful suspicion of stroke are referred to our tertiary hospital. Therefore, it is likely that acute MR imaging did not miss any clinically meaningful infarcts in these patients to the extent that such infarcts would warrant repeat neuroimaging.
A total of 145 patients underwent dedicated internal acoustic canal and inner ear imaging with heavily T2-weighted images (3D driven equilibrium radiofrequency reset pulse [DRIVE]). None of these images revealed any acute findings. One patient was diagnosed with acute labyrinthitis, but this was evident only on postcontrast T1-weighted images and not on T2-weighted images.
DISCUSSION
In this large-scale emergency MR imaging study, we found a prevalence of acute ischemic strokes in roughly 1 in 6 patients imaged for dizziness or vertigo. Nearly 1 in 10 patients had other clinically significant findings, whereas in 3 of 4 patients, MR imaging was unremarkable for acute pathology. Risk scores had only moderate performance in predicting any significant pathology or acute ischemic stroke. Isolated dizziness had no discriminative power concerning imaging outcomes. Dedicated internal acoustic canal and inner ear imaging had no role in the acute setting. CT had a low diagnostic yield among patients who had a stroke on MR imaging. Acute dizziness and vertigo remain challenging even when emergency MR imaging is readily available.
Overall, patients who had acute ischemic stroke were characterized by older age (generally older than 55 years of age), male sex, and high prevalences of cardiovascular risk factors and neurologic signs; patients with nonischemic significant pathology, by a high prevalence of headache and longer symptom duration; and patients with no significant pathology, by a high prevalence of vertigo. History-taking and proper clinical examination still play an important role when referring patients for MR imaging, because the aforementioned factors have a considerable impact on imaging outcomes.
We found several statistically significant associations between clinical variables and imaging outcomes that are consistent with those from the existing literature. Kabra et al18 reported an acute pathology rate of around 38% on early MR imaging and several stroke predictors (age older than 50 years, a high number of cardiovascular risk factors, a short duration of symptoms, and at least 1 neurologic sign). Machner et al22 reported a 24% acute pathology rate (varying between 0% and 50% among clinically defined subgroups) among emergency patients with dizziness who underwent adequate neuroimaging (early CT or delayed MR imaging). They documented hypertension, high ABCD2 scores, and any central oculomotor sign or focal abnormality that increased the risk of acute lesions. Similar to our analysis, they noted that among patients with vertigo (spinning sensations), acute lesions were less likely. Our findings further corroborate the use of MR imaging among patients with older age, cardiovascular risk factors, or neurologic signs.
Our risk scores reached moderate performances. The predictive model for acute strokes had an ROC AUC of 0.75 with an NPV of 90%, while the model for all significant pathology was slightly less accurate. We found no previously published risk scores for emergency MR imaging. In a study with 188 patients, Kabra et al18 demonstrated similar individual predictors of stroke (age, symptom duration, neurologic signs, and cardiovascular risk factors), each having NPVs of around 88%–90%.
The most common infarct location was the cerebellum. Notably, 25% of infarcts were located elsewhere than in the cerebellum or the brainstem. According to a recent connectivity-based analysis, supratentorial brain regions involved in the brain vertigo network include the bilateral insula, somatosensory cortex, higher-level visual areas, cingulate sulcus, and thalamus.23 Only 36% of prior CTs were positive for AIS, corroborating the role of acute MR imaging in detecting AIS in patients with vertigo. Missing an acute stroke may have serious adverse effects, such as predisposing the patient to a high risk of future, potentially more severe infarcts that could otherwise have been prevented, or, potentially, fatal secondary complications of the current stroke, such as brainstem compression and obstructive hydrocephalus.18,24
While its accuracy is considered superior to CT, even MR imaging does not have perfect sensitivity in the early detection of an AIS.25 In fact, DWI has been previously reported to be false-negative within the first 48 hours in up to 50% of small ischemic strokes in the posterior fossa.26 In our follow-up analysis of patients with NS, we concluded that acute MR imaging likely did not miss any clinically meaningful infarcts in our cohort. Modern MR imaging technology likely has a substantial sensitivity in detecting small infarcts in the posterior fossa and elsewhere in the brain, as was shown in the current study.
This study has 2 major strengths. First, we had a large sample size due to the routine use of emergency MR imaging in the emergency radiology department.21 A large sample size affords adequate statistical power to discern clinically meaningful effect sizes. Second, we used a data-driven approach by querying the referrals for specific symptoms instead of relying on diagnosis codes. This approach mitigates sampling bias because all patients with vertigo will be included irrespective of the final diagnosis. This imaging phenotypical approach is likely more proximal to the underlying biology than diagnosis codes. The present study represents a true clinical situation and offers a real-world overview of emergency patients with dizziness and vertigo. The fact that isolated dizziness (no other symptoms) had no significant discriminatory power suggests that the liberal inclusion of patients with various other symptoms did not significantly bias our results.
Yet, this study is limited by its retrospective and single-center design. Some referrals may have been incomplete or imprecise; therefore, the true prevalence of risk factors may have been underestimated. In addition to specific symptoms, relevant comorbidities and medical history may have been missing. The quality of the clinical note-keeping for each patient (reflecting real-world practice) determined the quality of the clinical data included in the present study. Classifying findings into NS and S groups was based on expert opinion and may, therefore, have been biased. In the classification, we used a consensus method among neuroradiologists and did not record interobserver agreement. The lack of relevant data may have contributed to the performance of the risk scores. In addition, the risk scores require prospective validation before claims of clinical utility can be made. The inclusion of patients with symptoms highly indicative of stroke (aphasia, ataxia, dysphagia) may have contributed to the higher diagnostic yield because these patients may be more likely to undergo neuroimaging regardless of having dizziness. We did not use the combination of axial and coronal DWI, which has been shown to have improved diagnostic accuracy for brainstem infarcts.27 Regarding generalizability, the present study is limited because we did not include acutely dizzy patients not scheduled for emergency MR imaging. Therefore, we do not know the factors that contributed to the need for emergency MR imaging perceived by the referring physician. We are not able to estimate the proportion of these patients undergoing first-line MR imaging. Most important, emergency MR imaging is not routinely available in all institutions, limiting the generalizability of our findings.
These results provide novel information on the diagnostic yield in this patient group when emergency MR imaging is readily available and commonly used in the emergency radiology department. Regarding the clinical value of emergency MR imaging findings, MR imaging likely altered the clinical management of patients with newly discovered neurologic disorders such as cerebrovascular (including acute infarction), demyelinating, and infectious diseases. Although the rate of nonsignificant pathology may seem too high (75%), ruling out infarctions with high sensitivity in these patients is likely valuable for them and their physicians. Most important, isolated dizziness lacked discriminative power on imaging outcomes because 14% of these patients had AIS on MR imaging.
CONCLUSIONS
Predictive modeling for including or excluding acutely dizzy patients for emergency MR imaging remains challenging. Because we were unable to reliably exclude patients who would not benefit from MR imaging, a relatively low threshold for ordering imaging to avoid misdiagnosis may be warranted. One in 4 patients had acute pathology on MR imaging. Predictors of acute pathology (older age, male sex, cardiovascular risk factors, and neurologic signs) may help to apply emergency neuroimaging more effectively among these patients, thus optimizing both the yield and clinical impact of emergency neuroimaging. Low diagnostic yields of CT and internal acoustic canal MR imaging sequences may offer an opportunity to reduce health care expenditures in the future.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received November 27, 2023.
- Accepted after revision January 19, 2024.
- © 2024 by American Journal of Neuroradiology