Abstract
Summary: To our knowledge, we are the first to report the diffusion-weighted MR imaging findings in a 15-day-old neonate with nonketotic hyperglycinemia. We found bilaterally symmetrical lesions of restricted diffusion in the dorsal brain stem, cerebral peduncles, and posterior limbs of the internal capsule, which were more conspicuous and extensive on diffusion-weighted MR images than on T2-weighted images. These lesions are in the myelinated tracts of the neonate and are compatible with the expected sites of abnormality in vacuolating myelinopathy.
Nonketotic hyperglycinemia (NKH) is an autosomal recessive inborn error of metabolism due to a defect in the glycine cleavage system. The elevated levels of glycine in the brain and CSF cause neurologic impairment, which in most present early in neonatal life with encephalopathy. Prognosis is poor, and most patients die in the first few weeks of life. Those who survive have intractable seizures and poor neurodevelopmental outcomes.
Most reports of diffusion-weighted MR imaging in neonatal brain are in hypoxic-ischemic encephalopathy and neonatal strokes. Increasingly, there are reports of the use of diffusion-weighted MR imaging in other causes of neonatal encephalopathy including metabolic diseases (1–3). We report a case of neonatal NKH whereby conventional MR imaging and diffusion-weighted MR imaging were performed at 15 days of life. To our knowledge, signal intensity abnormality on diffusion-weighted MR images has never been reported in neonatal NKH.
Case Report
A baby girl, of nonconsanguineous parents, was born by elective lower caesarean section at term with normal Apgar scores (9 at 1 minute; 10 at 5 minutes) and birthweight (3325 g). At 6 hours of life, she was found to have recurrent apnea, shallow breathing, depressed consciousness, and generalized hypotonia. Her neurologic status deteriorated, requiring mechanical ventilation at 24 hours of life, and she developed hiccups and twitching movements involving all four limbs.
The diagnosis of NKH was made by biochemical findings of elevated CSF, blood, and urine glycine levels, which were 388 μmol/L (normal range 1–15 μmol/L), 1619 μmol/L (normal range 230–740 μmol/L), and 9861 μmol/mmol creatinine (normal range 283–1097 μmol/mmol creatinine), respectively. The CSF-to-plasma glycine ratio was also elevated to 0.24 (normal range, 0.02–0.03). Ketoacidosis and organic acidemia were excluded. EEG showed a burst suppression pattern with occasional interhemispheric asynchrony, multifocal sharp waves and spikes, and absence of state changes after noxious stimuli, consistent with NKH.
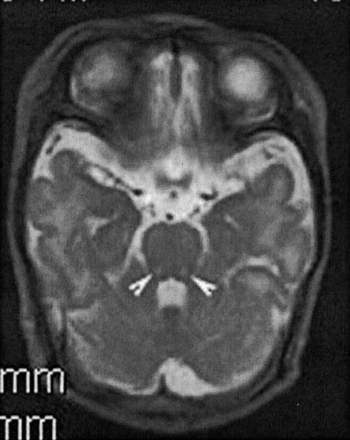
Conventional MR imaging and diffusion-weighted MR imaging (100/10,000/1000 [TE/TR/b factor]) was performed at 15 days of life. On conventional T2-weighted imaging, there were bilaterally symmetrical hyperintense foci in the dorsal midbrain and pons (Fig. 1 A and B). On diffusion-weighted MR images, these lesions were hyperintense in keeping with restricted diffusion (Fig 1C and D) and, in addition, bilaterally symmetrical hyperintense lesions were noted in the cerebral peduncles (Fig 1C) and along the posterior limbs of the internal capsule (Fig 1G). The sites of restricted diffusion demonstrated corresponding reduction in apparent diffusion coefficient (Fig 1E, F, H). The lesions were more conspicuous and extensive with diffusion-weighted MR imaging than T2-weighted imaging (Fig 1H). The ventricles were normal in size, and there was no periventricular white matter signal intensity abnormality. The corpus callosum was hypoplastic. The cerebellum and posterior fossa were normal in appearance.
Patient 1, a 15-day-old neonate who presented with encephalopathy.
Axial fast spin-echo T2-weighted images (140/3800/2[TE/TR/NEX]) of the midbrain (A) and pons (B) show hyperintense lesions in the dorsal midbrain and pons (arrows). Axial diffusion-weighted MR images (100/10000/1000 [TE/TR/b factor]) of the midbrain (C) and pons (D) taken at the same levels show hyperintense lesions of restricted diffusion. The lesions are more conspicuous than on T2-weighted images, and additional lesions of restricted diffusion are seen in the bilateral cerebral peduncles (C, arrowheads). Susceptibility artifact is noted in the right frontal region from an ECG lead. Apparent diffusion coefficient maps show corresponding reduced apparent diffusion coefficient in the midbrain (E) and pons (F). Axial diffusion-weighted MR image (G) and apparent diffusion coefficient map (H) show restricted diffusion in the posterior limbs of the internal capsules (arrows). Susceptibility artifact is noted in the right frontal region from an ECG lead. Region of interest measurements of the posterior limbs of the right and left internal capsule show reduction of apparent diffusion coefficients to 0.87 μm2/msec and 0.97 μm2/msec, respectively. No signal intensity abnormality is seen in the corresponding fast spin-echo T2-weighted image (I).
Although treatment with dextromethorphan was started once the diagnosis was made, her condition continued to deteriorate, and she died at 23 days of life.
There was, incidentally, another incidence of neonatal death in the family 3 years before. The patient’s elder sister suffered a similar clinical course after birth and died at 40 days of life. Retrospective evaluation of the MR imaging performed at day 7 of life revealed similar hyperintensities in the dorsal brain stem on T2-weighted images (Fig. 2). Diffusion-weighted MR imaging was not performed at that time. No definitive diagnosis was made, although it is likely that she also succumbed to neonatal NKH.
A 7-day old neonate, the older sister of patient 1, also presented with neonatal encephalopathy.
Axial fast spin-echo T2-weighted image (130/4200/1[TE/TR/NEX]) of the midbrain shows similar hyperintense lesions in the posterior midbrain (arrowheads).
Discussion
Previous reports of imaging abnormalities in NKH during the neonatal period are structural and include ventriculomegaly, absent corpus callosum, and posterior fossa cysts (4–6). At 4.5 months of age, absent myelination in the internal capsule and dorsal brain stem was noted in one published report (6). Atrophy and decreased or absent myelination of the supratentorial white matter have been reported later in infancy (4, 6, 7). Recently, it has been shown that an abnormal glycine peak (3.55 ppm) can be detected by proton MR spectroscopy in NKH (8, 9).
In our patient, the sites of abnormal signal intensity are confined to the white matter tracts that are myelinated at birth. These are in the dorsal pons, midbrain, cerebellar white matter, posterior limb of the internal capsule, lateral thalamus, and globus pallidus. The pathology of NKH is that of vacuolating myelinopathy whereby myelinated areas undergo vacuolation, demyelination, and astrocytosis (10). Vacuolating myelinopathy is found in several metabolic diseases, including Canavan disease, maple syrup urine disease, proprionic acidaemia, van der Knaap leukoencephalopathy, some mitochondrial diseases, and intoxications (3, 10). In the neonate, the distribution of the lesions is characteristic, because the presence of formed myelin sheaths is a prerequisite for the development of vacuolating myelinopathy (10). In maple syrup urine disease with neonatal presentation, swelling and high signal intensity on T2-weighted imaging has been described in sites similar to those noted in our patient (10, 11).
Restricted diffusion has been reported in the acute and subacute stage of other vacuolating myelinopathies, including Canavan disease (1), and in a case of intoxication (heroin-induced spongiform leukoencephalopathy; 12). These lesions were in the supratentorial white matter of a 17-month old child and an adult, respectively. On electron microscopy, intramyelinic vacuole formation and splitting of myelin lamellae are observed in vacuolating myelinopathies (10). In addition, there is swelling of the astrocytes with enlarged nuclei and watery cytoplasm (10). It has been proposed that restricted diffusion can be attributed to the accumulation of fluid between the layers of myelin lamellae. In reports of van der Knapp leukoencephalopathy, the reverse was found to occur, with lesions of increased apparent diffusion coefficient instead. Gelal et al suggest that enlarged extracellular spaces, possibly by the coalescence of small vacuoli in a later stage of disease, may account for this (3). It is not known whether this will occur in the later stage of NHK.
It is interesting to note that previous MR imaging reports of NKH performed during the early neonatal period have never mentioned similar hyperintense dorsal brain stem lesions on T2-weighted images (4, 8, 9). These lesions may therefore be apparent only during a certain phase of the pathologic process, or they may not occur consistently in NKH neonates. Also, because these lesions can be subtle on T2-weighted images, they may have been missed.
Conclusion
This case illustrates a pattern of vacuolating myelinopathy in a neonate with NKH and highlights the role of diffusion-weighted MR imaging in increasing lesion conspicuity, characterizing regions of different diffusion activity and, possibly, early detection of lesions.
References
- Received August 28, 2002.
- Accepted after revision October 31, 2002.
- Accepted after revision October 31, 2002.
- Copyright © American Society of Neuroradiology