Abstract
Summary: Benign primary intraosseous meningioma presenting with osteolytic skull lesion and soft-tissue component is rare. CT and MR imaging of a patient with frontoparietal scalp swelling showed an osteolytic intracalvarial lesion with an extradural soft-tissue component. Following wide surgical resection, the histological examination revealed an intraosseous chordoid meningioma. The clinical and radiological findings of primary intraosseous meningioma are discussed and the relevant literature is reviewed.
Primary calvarial meningiomas are uncommon, and to our knowledge, the chordoid type intraosseous meningioma has not been previously reported. We present a patient with primary calvarial meningioma and review the literature on this subject.
Case Report
A 44-year-old man presented with a scalp swelling of the right frontoparietal region. The scalp swelling was present for the last 8 years and had gradually increased in size. The swelling was not painful, and there was no history of trauma.
Physical examination revealed a swelling of the right frontoparietal region about 3 cm in diameter, which was not adhering to the overlying skin. The swelling was not mobile or tender. The patient had no neurologic deficit. The laboratory studies were unremarkable. Radiographs of the skull revealed a well-defined area of osteolysis in the right frontoparietal region (Fig 1). CT revealed a right-sided frontoparietal intradiploic mass expanding the calvaria with prominent bone destruction (Fig 2A). The lesion extended through the skull defect both intra- and extracranially. The mass lesion showed prominent enhancement following intravenous contrast injection (Fig 2B). Three-dimensional reformatted CT (Fig 2C) clearly demonstrated the osteolytic skull lesion. MR imaging showed a solitary calvarial mass lesion of the frontoparietal region that was hypointense on T1-weighted and hyperintense on T2-weighted images (Fig 3A,-B). The T1-weighted imaging following gadolinium injection demonstrated the prominent and homogeneous enhancement of the lesion (Fig 3C). MR imaging clearly revealed the intracranial but extradural extension of the lesion. Preoperative diagnosis was a primary or secondary malignant osteolytic lesion.
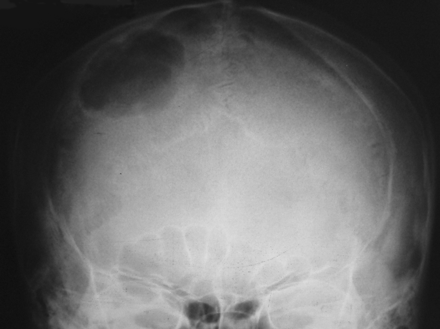
Plain skull radiograph shows a well-defined solitary lytic lesion in the right frontoparietal region.
CT scan with bone window (A) demonstrates a right-sided frontoparietal intradiploic mass expanding the calvaria with cortical destruction. Postcontrast CT scan (B) shows prominent enhancement and intracranial extension of the lesion. Three-dimensional reformatted CT image (C) clearly demonstrates the osteolytic skull lesion.
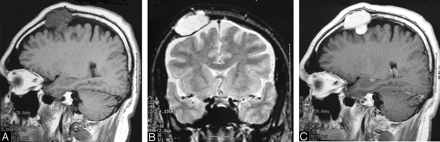
Sagittal T1-weighted (A) and coronal T2-weighted (B) MR images show the frontoparietal intracalvarial mass lesion that was hypointense on T1-(A) and hyperintense on T2-weighted (B) images. The lesion shows intense and homogeneous enhancement on postcontrast T1-weighted (C) image. MR images (A–C) reveal the intracranial extension and extradural location of the lesion.
A search was made for a possible primary to rule out a calvarial secondary deposit; however, no primary could be found. At surgery, the scalp could be easily elevated from the tumor, which had caused destruction of both the inner and outer tables of the skull and had extended extradurally. The tumor and the surrounding bone were removed, followed by cranial reconstruction. There was no intradural extension of the lesion. Histologically, the tumor, which showed bone infiltration, was diagnosed as chordoid meningioma because it contained regions with trabeculae or ribbons of eosinophilic vacuolated cells in a myxoid background (Fig 4). Histologically, there were no features to suggest sarcomatous changes. The patient has been well for 1 year following the operation with no evidence of recurrence.
Photomicrograph of the tumor shows the chordoid meningioma with eosinophilic vacuolated tumor cells (large arrow) in a mucous-rich matrix (small arrow) (hematoxylin and eosin, original magnification ×200).
Discussion
Extracranial meningiomas are rare; the reported incidence is 1%–2% of all meningiomas (1, 2). The meningiomas arising in locations outside the dural compartment have been called ectopic, extradural (epidural), calvarial, cutaneous, extracranial, extraneuraxial, or intraosseous meningiomas. To avoid the confusion in nomenclature, Lang et al (3) has proposed a single term, “primary extradural meningioma” for such lesions. This term highlights the origin of these tumors as being separate from the dural coverings of any part of the brain or spinal cord and further differentiates these meningiomas from “primary intradural meningiomas,” which may have secondary extracranial extensions and/or may have metastasized (3). Although some authors have emphasized that this group of meningiomas should have no connection to the dura mater or any other intracranial structures (4, 5), other reports include tumors with intracranial growth (6–8). In our opinion, if the tumor is invading only the outer layer of the dura and its main mass is extracranial, as in this case, it should be accepted as primary extradural meningioma.
Primary extradural meningiomas are classified as purely extracalvarial (type I), purely calvarial (type II), or calvarial with extracalvarial extension (type III). According to the site of location of the tumor, Lang et al (3) further subdivided type II and type III lesions into convexity (C) or skull base (B) forms. Because of the extracalvarial extension, the case presented here is a type IIIC meningioma.
Many different hypotheses exist regarding the origin of primary calvarial meningiomas. They are thought to arise from ectopic meningocytes or arachnoid cap cells trapped in the cranial sutures during molding of the head at birth (9). Although the case presented here supported this hypothesis with its close relationship to the coronal suture, only 8% of the reported calvarial meningiomas were found in relationship with a cranial suture (3). Misplacement and entrapment of meningothelial cells into suture or fracture lines as a result of trauma has also been speculated as the probable cause of calvarial meningioma (10). In the literature, only 5 of the 36 cases of primary intraosseous meningioma had a history of trauma to the head in the region of subsequent tumor development (5). In our case, there was no definite history of trauma or radiographic manifestation of an old skull fracture. Meninges are mesenchymal in origin; meningiomas may develop from multipotential mesenchymal cell precursors, thereby accounting for the occurrence of meningiomas in uncommon locations (11). Cutaneous meningiomas could be congenital in origin, in which case they arise from arachnoid cell rests located in the skin as a result of defective closure of the neural tube wherein the meningeal tissue is pinched off into the surface (12). They are also thought to arise from multipotent mesenchymal cells as a reaction to an unidentified stimulus (11).
According to the literature, 68% of the primary extradural meningiomas involved the calvaria (3). Frontoparietal and orbital regions are the most common locations for intraosseous meningiomas. Other sites reported in the literature are the subcutaneous tissue of skin, the paranasal sinuses, the nasal cavities, oral cavities, parapharyngeal space, neck, salivary glands, and along the perineural sheath of the cranial nerves. Rare meningiomas occurring in the lung, mediastinum, adrenal gland, paraspinal region, and even in the finger have also been reported (3). Although primary intradural meningiomas occur twice as frequently in women as in men, primary extradural meningiomas occur with approximately the same frequency in each sex (3). Whereas primary intradural meningiomas and primary extradural meningiomas occur predominantly during later decades of life, primary extradural meningiomas also have a second peak incidence in younger patients (especially during the second decade) (3). Primary extradural meningiomas have a wider range of clinical presentations than primary intradural meningiomas, primarily due to variety of locations. As in our case, patients with calvarial intraosseous meningiomas typically present with slow-growing scalp swelling that may or may not be painful. They do not show any neurologic symptoms or signs, unless the lesion extends through the inner table and compresses intracranial structures. These lesions may be asymptomatic and detected incidentally (13).
Biologically, calvarial meningiomas have been observed to be benign and slow-growing. On the other hand, calvarial meningiomas are more prone to develop malignant changes (11%) compared with intracranial meningiomas (2%) (3, 11). Bone remodeling and calvarial thickening at the site of origin of the meningioma are frequent with these tumors. Osteolytic skull lesions are also known to occur in calvarial meningiomas. In the review of the literature, though hyperostosis was the most common radiographic finding (reported in 59% of the cases) osteolysis, as in our case, was reported in 35% and a mixed picture of hyperostosis and osteolysis was reported in 6% of the cases (5). Meningiomas presenting with scalp swelling, osteolytic skull lesions, and extracranial soft-tissue masses are more aggressive in nature than others (14–16). Younis and Sawaya (17) reported 3 patients who presented with scalp swellings, osteolytic skull lesions, and extracranial soft-tissue masses. All of their patients had malignant meningiomas. On the basis of their experience, they contended that osteolysis, when associated with a soft-tissue mass, is a strong reason to suspect a malignant meningioma (17). Hussaini (18) had also reported a case with a lytic lesion in the frontal region that was diagnosed as malignant meningioma histologically. Partington et al (19) reported a case that presented with a soft-tissue mass and an osteolytic skull lesion that turned out to be an atypical meningioma. This tumor was also found to secrete carcinoembryonic antigen. Although there were reported cases (14) that presented with osteolytic skull lesions and soft-tissue masses diagnosed as benign histologically as in our case, osteolytic meningiomas associated with soft-tissue components should be considered “malignant until proved otherwise” (19).
Conventional radiographs are usually of limited value in the diagnosis of calvarial meningiomas because of the superimposed bony structures. CT with bone windows is necessary in the detection of the tumor, cortical destruction, and both intra- and extraosseous extension. MR imaging provides a better anatomic delineation in the evaluation of the soft-tissue component and extradural extension of the lesion. The differential diagnosis for osteolytic intraosseous meningioma includes metastatic cancer, plasmacytoma, giant cell tumor, hemangioma, epidermoid cyst, osteogenic sarcoma, eosinophilic granuloma, aneurysmal bone cyst, or fibrous dysplasia. Although rare, intraosseous malignant meningioma should be excluded in an osteolytic skull lesion with an associated soft-tissue component.
Chordoid meningioma is a rare variant of meningioma that shows a striking histologic resemblance to chordoma. In our case, the histologic examination of the surgical specimen revealed a tumor composed of trabeculae or ribbons of eosinophilic vacuolated cells embedded in a mucous-rich matrix. Although the location of chordoid meningiomas was similar to that of other types of meningiomas, to the best of our knowledge, chordoid-type intraosseous calvarial meningioma has not been previously reported.
In symptomatic primary intraosseous meningioma, total tumor removal with a wide surgical resection followed by cranial reconstruction is the treatment of choice. Because our case was symptomatic and suggestive of malignancy, the decision to operate was made and the total removal of the tumor was managed. If only subtotal resection is possible because of involvement of critical structures within the orbit, paranasal sinuses, or skull base, the residual tumor should be followed radiologically (5). Adjuvant radiation therapy is recommended in patients whose residual lesions are symptomatic and/or show evidence of progression (5). Because chordoid meningiomas are World Health Organization grade II tumors that have a potential to recur, radiologic follow-up is very important.
In conclusion, although rare, a primary intraosseous meningioma should be considered and malignant meningioma should be excluded in patients with osteolytic calvarial lesions associated with a soft-tissue component. CT and particularly MR imaging are the radiologic techniques that are inevitable in the diagnosis and evaluation of the soft-tissue component and extradural extension.
References
- Received October 20, 2004.
- Accepted after revision October 29, 2004.
- Copyright © American Society of Neuroradiology