Abstract
BACKGROUND AND PURPOSE: Therapeutic hypothermia has reduced morbidity and mortality and is associated with a lower burden of lesions on conventional imaging in NE. However, its effects on brain microstructure and metabolism have not been fully characterized. We hypothesized that therapeutic hypothermia improves measures of brain microstructure and metabolism.
MATERIALS AND METHODS: Forty-one neonates with moderate/severe NE (29 treated with hypothermia, 12 nontreated) and 12 healthy neonates underwent MR imaging, DTI, and 1H-MR spectroscopy. MR imaging scans were scored by the predominant pattern of brain injury: normal, watershed, and BG/thalamus. ADC, FA, Lac:NAA, and NAA:Cho values from bilateral BG and thalamus ROIs were averaged. T test and linear regression analysis were used to determine the association between hypothermia and MR imaging quantitative measures.
RESULTS: Conventional MR imaging findings were normal in 41% of treated neonates; all nontreated neonates had brain injury. Values of MR imaging metrics were closer to normal in treated neonates compared with nontreated neonates: ADC was 63% higher in the BG and 116% higher in the thalamus (both P < .05), and Lac:NAA was 76% lower (P = .04) in the BG. Treated neonates with normal MR imaging findings had normal 1H-MR spectroscopy metabolites, and ADC was higher by 35% in the thalamus (P = .03) compared with healthy neonates.
CONCLUSIONS: Therapeutic hypothermia may reduce disturbances of brain metabolism and preserve its microstructure in the setting of NE, possibly by minimizing cytotoxic edema and cell death. Long-term follow-up studies are required to determine whether early post-treatment DTI and 1H-MR spectroscopy will be useful biomarkers of treatment response.
ABBREVIATIONS:
- BG
- basal ganglia
- CI
- confidence interval
- FA
- fractional anisotropy
- Lac
- lactate
- NE
- neonatal encephalopathy
- WS
- watershed
Therapeutic hypothermia is increasingly being adopted as the standard treatment for NE secondary to presumed hypoxia-ischemia because multiple randomized trials have demonstrated a reduction in mortality and disability.1⇓⇓–4 Hypothermia is most beneficial in those with moderate NE, but the neuroprotection conferred is incomplete, with only an 11% reduction in death or disability, so the search for adjunctive therapies to improve on the benefit of hypothermia continues.5 MR imaging is a robust predictor of neurodevelopmental outcome in both hypothermia-treated and nontreated neonates with NE.6⇓⇓–9 Compared with nontreated neonates, those who receive treatment are more likely to have normal conventional imaging findings at the completion of therapy.8,10 Regardless of therapy, injury to the deep gray nuclei, as assessed by using conventional qualitative and advanced quantitative MR imaging techniques, has a strong association with adverse outcome.9,11 Understanding the impact of hypothermia on MR imaging parameters is necessary because both qualitative and quantitative MR imaging techniques will be used as early biomarkers of outcome in future trials of neuroprotection.12
Advanced MR imaging techniques, such as DTI and 1H-MR spectroscopy, are important adjuncts in the study of brain injuries associated with NE because they can detect and quantify abnormalities that may be too subtle to detect by conventional MR imaging alone. DTI can detect changes in random water motion caused by alterations of tissue microstructure. Parameters typically used to measure these alterations include FA, an indicator of directionality of water motion (and indirectly of axonal organization) and ADC, the average magnitude of water motion during each DTI acquisition. 1H-MR spectroscopy can characterize the metabolic stress associated with hypoxia-ischemia. Measured metabolites include the following: Lac, a marker of anaerobic metabolism; NAA, a marker for neuronal activity; and Cho, a marker of membrane turnover.13,14 Following hypoxia-ischemia, there are characteristic changes in FA, ADC, and metabolite levels. There is an acute drop in both ADC and FA values, followed by a period of pseudonormalization of ADC values, which occurs around the second week after birth (7–10 days of life).15⇓⇓–18 Changes in metabolic ratios (elevated Lac:NAA, and reduced NAA:Cho) can be detected during the first 2 days of life and continue to worsen for 4–5 days. In neonates with NE who did not receive therapeutic hypothermia, both DTI and 1H-MR spectroscopy findings of the deep gray nuclei were strongly associated with outcome.19,20 Both DTI and 1H-MR spectroscopy are under evaluation as possible early biomarkers to assess response to neuroprotective therapies and as surrogate markers for long-term neurodevelopmental outcome.12
The goal of this study was to evaluate DTI and 1H-MR spectroscopic metrics in the deep gray nuclei in neonates with encephalopathy treated with therapeutic hypothermia. We tested the following hypotheses: Therapeutic hypothermia results in improved measures of diffusivity and metabolism; in neonates with predominant qualitative injury to the deep gray nuclei, treatment is associated with improved measures of diffusion and metabolism; and treated neonates with normal conventional imaging findings have diffusion and metabolic parameters similar to those in healthy neonates without brain injury.
Materials and Methods
Human Subjects
The Committee on Human Research approved the MR imaging research protocol and review of clinical data collected prospectively by trained neonatal research nurses. Written informed consent was obtained from the parents of all neonates in the present study.
Clinical Data
Forty-one term neonates with moderate-to-severe encephalopathy secondary to presumed hypoxia-ischemia and 12 healthy term neonates who underwent MR imaging, DTI, and 1H-MR spectroscopy within 2 weeks of birth were selected for the present study from an ongoing cohort study of MR imaging in neonates with perinatal asphyxia. Of the 41 neonates (all born from 2007 forward), 29 received therapeutic hypothermia, and 12 were eligible for hypothermia but were either born before its availability in our nursery or they were referred to our hospital outside of the therapeutic window (>6 hours of life). Conventional imaging findings in a subset of this cohort were previously reported.10 Therapeutic hypothermia was established as the standard of care at our center late in 2007. Our institutional guidelines, similar to those of the randomized trials, are as follows: 1) birth at ≥36 weeks postmenstrual age; 2) presence of ≥1 of the following: an Apgar score of <5 at 10 minutes of life, a history of prolonged resuscitation following birth, the presence of severe acidosis defined as cord pH or any arterial or venous pH <7.0 within 60 minutes of birth, or a base deficit greater than −12 from cord blood or arterial blood gas within 60 minutes of birth; and 3) the presence of moderate-to-severe encephalopathy identified by the attending neonatologist or pediatric neurologist. Neonates with congenital anomalies or presumed inborn errors of metabolism were excluded. For neonates born outside our center, passive cooling was performed during transport. Whole-body cooling was achieved with a blanket cooling device (Blanketrol III; Cincinnati Subzero, Cincinnati, Ohio) and regulated by the infant's core temperature measured with a rectal probe. Neonates were maintained at 33.5°C for 72 hours. During therapeutic hypothermia, all neonates were sedated with morphine administered as a continuous infusion to minimize shivering.
Clinical data reviewed for this study included sex, birth weight, gestational age at birth, Apgar scores, need for chest compressions at birth, umbilical arterial pH, severity of encephalopathy on presentation measured by an encephalopathy score, and age at scanning (Table 1). The encephalopathy score is based on alertness, feeding, tone, respiratory status, reflexes, and seizure activity (range is 0–6, where 0 = normal).21
Clinical characteristics and the distribution of brain injury patterns in neonates with encephalopathya
MR Imaging
All neonates underwent MR imaging studies on a 1.5 T GE HDx scanner (GE Healthcare, Milwaukee, Wisconsin). The scans were acquired by using a custom-built neonatal head coil in an MR imaging–compatible incubator22 with the following protocol: T1-weighted (3D spoiled gradient-echo; TR/TE = 36 ms/minimum; partition size = 1.5 mm; matrix = 256 × 192; 1 excitation), T2-weighted (dual-echo, spin-echo; TR/TE = 3 s/60 and 120 ms; section thickness = 4 mm; matrix = 192 × 256), DTI (spin-echo, single-shot echo-planar, TR/TE = 8000/99 ms; section thickness = 3 mm; matrix = 128 × 128; FOV = 30 cm, b = 700 s/m2; 30 directions for the patients with encephalopathy and 6 directions with 3 averages for the healthy neonates), and 3D lactate-edited MR spectroscopic imaging (8 × 8 × 8 at 1-mL resolution, TE/TR = 144 ms/1 s).
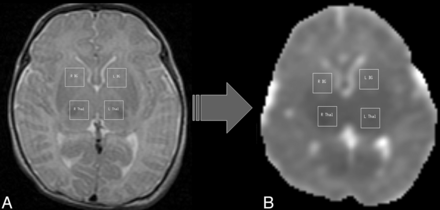
The total examination time was <1 hour. Regions of interest were drawn on T2-weighted images by using custom-built software in Interface Design Language (ITT VIS; http://www.exelisvis.com/language/en-us/productsservices/idl.aspx), bilaterally (1 cm × 1 cm2) for the BG and thalamus (Fig 1). Diffusion images were processed by using the FMRIB Software Library (http://www.fmrib.ox.ac.uk/fsl/).23 Built-in eddy current correction and masking by using the FA images were used. ADC and FA values were obtained from the BG and thalamus ROIs. The 3D MR spectroscopic imaging data were processed by using methods previously described.24 Peak-height ratios were generated for the entire brain and extracted for the specific ROIs for this study. Left and right values were averaged. Figures 1 and 2 demonstrate the ROIs from which the DTI and MR spectroscopic imaging values were measured.
Regions of interest were drawn on T2-weighted images and were later applied to diffusion maps. A, T2-weighted image (transaxial section) shows ROIs drawn bilaterally for the BG and thalamus (Thal) structures. B, ADC map shows the same ROIs applied onto the corresponding transaxial section. L indicates left side; R, right side.
1H-MR spectroscopy spectral array was generated for the entire brain and later extracted for the studied structures. T2-weighted image (transaxial section) shows the 1H-MR spectroscopy brain selected region grid and the corresponding spectral arrays generated (on the right). Peak ratios calculated from the sections covering the BG and thalamus (Thal) bilaterally were used for the study. L indicates left side; R, right side.
All scans were acquired during normothermia within 2 weeks after birth: treated neonates at median day 5 (P25 = 4; P75 = 6) after birth; nontreated neonates at day 4 (P25 = 3; P75 = 5); and healthy neonates at day 8 (P25 = 4; P75 = 10). T1, T2, and DWI sequences were scored by an experienced neuroradiologist blinded to the clinical history, by using a validated scoring system associated with neurodevelopment outcome.7 The predominant pattern of injury was determined as the following: normal, WS, or BG/thalamus. The WS pattern was assigned when the WS region scores were higher than the BG/thalamus scores. The BG/thalamus pattern was assigned when the BG/thalamus scores were higher than or as high as the WS region scores. Neonates with total brain injury (maximum BG/thalamus and WS scores) were assigned to the BG/thalamus pattern.
Statistical Analysis
Statistical analysis was performed by using STATA 9 (StataCorp, College Station, Texas). Clinical characteristics were compared between treated and nontreated neonates by using the Student t test for continuous variables, Wilcoxon rank sum test for nonparametric variables, and the χ2 for categoric variables. The Student t test and linear regression were used to compare MR imaging variables between the groups. We compared the following: 29 neonates treated with hypothermia versus 12 nontreated, 7 treated versus 6 nontreated neonates with BG/thalamus–predominant injury, and 12 treated neonates with normal conventional imaging findings versus 11 healthy neonates with normal conventional imaging findings. Because diffusion and spectroscopy values are known to change with time and there was a difference in the time of scanning between groups, linear regression with an interaction term for the time of scanning and treatment group was performed.
Results
Clinical Characteristics and MR Imaging Patterns of Injury
Treated and nontreated groups had similar sex distributions. Clinical characteristics including the severity of perinatal asphyxia (umbilical arterial cord pH) and the severity of encephalopathy at presentation were similar. Treated neonates were imaged, on average, 1 day later than nontreated neonates (P = .02) and were more likely to have normal conventional imaging findings; all in the nontreated group had brain injury identified on conventional MR imaging (P = .03). Two neonates with severe BG/thalamus injury died following withdrawal of life-sustaining measures (1 in the treated group and 1 in the nontreated group) (Table 1).
DTI and 1H-MR Spectroscopy Findings
Treated versus Nontreated Neonates.
Results of the univariate analyses are demonstrated in Table 2. Mean ADC values were 13%–16% higher in treated patients in both the BG and thalamus ROIs, and FA was 40% higher in the BG. 1H-MR spectroscopy showed differences in the BG, with average Lac:NAA 51% lower and NAA:Cho 17% higher in treated patients. There was interaction between age at scanning and treatment group on the mean ADC values measured in the BG (Fig 3). As the age at scanning increased, the difference in mean ADC values between treated and nontreated neonates decreased. Following adjustment for age at scanning and the interaction, the association between hypothermia and quantitative MR imaging measures persisted and the effect size was greater: In treated neonates, ADC values were 63% higher in the BG (95% CI, 26%–84%; P = .007) and 116% higher in thalamus (95% CI, 78%–130%; P = .003) and Lac:NAA was 76% lower in the BG (95% CI, 2.7%–273%; P = .04).
DTI and 1H-MRS findings in neonates with neonatal encephalopathya
ADC values in the basal ganglia and regression lines for each treatment group. ADC values and fitted regression lines show the mean ADC values in the basal ganglia for each treatment group and the interaction between treatment and age at imaging. As age at imaging increases, the difference in the mean ADC values between the groups decreases.
Treated versus Nontreated Neonates with BG/Thalamus–Predominant Injury.
The number of neonates in each group was small. Overall, no statistically significant differences were observed in the measures of diffusivity and metabolism. Although a significant difference was not detected, treated neonates with qualitative BG/thalamus–predominant injury had ADC values that were 20%–24% higher and mean Lac:NAA values that were 55%–60% lower (Table 3).
DTI and 1H-MRS findings in neonates with predominant qualitative injury to the basal ganglia/thalamusa
Treated Neonates with Normal Imaging Findings versus Healthy Neonates.
Univariate analysis (Table 4) demonstrated that treated neonates had higher ADC values (7%–12%) in both BG and thalamus ROIs and higher FA (32%) in the BG. The 1H-MR spectroscopy values in these 2 groups were similar for both Lac:NAA and NAA:Cho. When evaluated with linear regression, including terms for age at scanning and an interaction term for age at scanning with treatment, only the association between treatment and ADC values in the thalamus remained (mean ADC values higher by 35%; 95% CI, 5.2%–59%; P = .03).
DTI and 1H-MRS findings in neonates with normal conventional MRI findingsa
Discussion
The results of this study indicate that therapeutic hypothermia for NE is associated with improved brain metabolism and preserved brain microstructure in the deep gray nuclei. Therapeutic hypothermia is associated with higher ADC values in the basal ganglia and thalamus and lower Lac:NAA in the basal ganglia. Due to the strong association between injury to the deep gray nuclei and outcome, we attempted to evaluate differences in microstructure and metabolism between treated and nontreated neonates with predominant deep gray nuclear injury but were unable to demonstrate statistically significant differences between the groups. Finally, treated neonates with normal qualitative imaging findings had metabolic ratios similar to those in healthy neonates without brain injury; for reasons that are not yet established, their ADC values in the thalamus were slightly higher than those of healthy neonates.
Serial imaging studies of neonates with encephalopathy who did not receive hypothermia have characterized sequential changes in ADC, FA, and metabolic ratios.15⇓⇓–18 As shown in the initial study, soon (when imaged at 2–5 days) after the latent period (which lasts 6–15 hours), there is a drop in both ADC and FA values thought to be secondary to cytotoxic edema and metabolic stress related to secondary energy failure, which may dramatically affect water diffusion. This is followed by a period of pseudonormalization of ADC values, which occurs around the second week (7–10 days) after birth.15⇓⇓–18 Analysis of metabolic parameters reveals decreased NAA:Cho and increased Lac:NAA ratios.14,19 These changes are presumed to be secondary to mitochondrial dysfunction, decreased intracellular energy, neuronal loss with cell membrane turnover, and possibly astrocytic injury.25 In the hypothermia-treated neonates, we did not observe the expected decrease in ADC and NAA or the expected increase of lactate in the deep gray nuclei.
While the precise mechanism of neuroprotection conferred by hypothermia is not fully understood, it is likely multifactorial with different mechanisms specific for each phase of cerebral injury. The possible mechanisms were presented in a recent review by Drury et al.26 This review highlights the possibility that the degree of neuroprotection may be related to the timing of initiation of therapy. Hypothermia may have a discrete impact on each of the stages that follow a hypoxic-ischemic insult (acute insult, reperfusion [30–60 minutes postinsult], latent period [6–15 hours postinsult], and secondary energy failure [6 hours to >3 days]). In the present study, hypothermia was initiated early, with most neonates reaching the target temperature at <6 hours after birth, but the precise timing of treatment initiation related to the acute insult is not known.
Despite this uncertainty, the data support the hypothesis that hypothermia results in recovery of oxidative metabolism (less lactate detected in treated neonates), decreased cytotoxic edema (ADC levels near normal), and decreased neuronal injury (more normal NAA) as measured by these advanced imaging techniques. The clinical significance of and the reasons for higher ADC values within the cerebrum following hypothermia therapy are unknown. Possibly the findings are transient and related to hypothermia therapy itself, though the subjects were imaged when they were normothermic. Another possibility would be that the hypothermia merely delays secondary energy failure; however, prior studies have shown that therapeutic hypothermia is effective in improving outcome in the short-term (18 months of age),1 indicating that the effects are not merely temporary. Finally, we cannot entirely exclude the possibility that the increase in ADCs may be technical and related to the increased number of directions of data acquisition in the hypothermia-treated neonates compared with controls.27,28 This explanation seems unlikely, however, because ADC values tend to decrease by approximately 5% with an increasing number of directions from 6 to 30, but we noted an increase in ADC values. A study of serial DTI and MR spectroscopy during therapy, immediately after therapy, and several days after rewarming may better elucidate the impact of hypothermia on DTI and MR spectroscopy parameters.
There are few studies in human neonates that report associations between hypothermia and brain microstructure or metabolism. The data presented here differ from the published studies in objectives, timing of imaging, and study population. One small study, comparing 3 hypothermia-treated neonates with 3 nontreated and 4 healthy controls, showed that mean diffusivity in the putamina and thalami of treated neonates was similar to that seen in control infants, but the timing of imaging was quite variable, with some as early as the first week of life and others as late as 7 weeks after birth, making a direct comparison with our study difficult.29
A second study of a larger cohort (n = 47) imaged at 5–12 days of age evaluated the relationship among ADC values, T1 and T2 signal intensity ratios, and outcomes at discharge and at 9 months of age.30 All subjects in this study received hypothermia, so there was no comparison between treated and nontreated neonates. The authors reported no difference in ADC values between neonates with a normal or mild deficit and those with a severe deficit (death or abnormal consciousness, tone, hearing, vision, absent gag/suck/feeding autonomy) when evaluated at discharge or 9 months of age. They did find that T2 intensity ratios were independently associated with outcome but were not better at predicting outcome than qualitative measures. The inability of ADC values to differentiate among neonates with different outcomes was likely related to the timing of the MRI because the scans were obtained at a median age of 7 days, likely during the phase of pseudonormalization of the ADC values. Finally, in the most recent single-center study, 10 of 81 study subjects were treated with hypothermia.20 As a secondary aim, the authors compared the ADC values and Lac:NAA ratios between hypothermia-treated and nontreated neonates. ADC values in the basal ganglia were similar in treated and nontreated neonates with a favorable long-term outcome. A direct comparison of the ADC values and metabolic ratios between treatment groups was not performed. The authors also demonstrated that when imaging is performed during the first week of life, adding ADC or Lac:NAA ratios to qualitative scoring resulted in better prediction of outcome than qualitative scoring alone.
This study has several limitations. Major limitations include the small sample size, especially in the nontreated group with NE and in the number of subjects with predominant injury to the deep gray nuclei, as well as a lack of long-term neurodevelopmental follow-up data. This small sample size may have limited the ability to detect a difference between the groups. Follow-up data in this cohort will be required to understand the very important question of clinical relevance of DTI and MR spectroscopy values after hypothermia; this answer will determine whether the MR imaging metrics will be useful as early biomarkers of treatment efficacy. Another important limitation is the timing of MR imaging. ADC values are known to evolve with time, yet nontreated subjects were imaged slightly earlier than treated subjects, possibly before pseudonormalization and healthy neonates were imaged later than the other 2 groups. This difference in the timing of MR imaging studies, in addition to the treatment-related factors, may contribute to the difference in ADC values. This difference was accounted for by adjusting for the time of scanning and by including an interaction factor between treatment and age at scanning because both have an impact on the ADC values that are measured.17,18 The difference persisted after adjustment, making it less likely that the results are due to differences in the timing of imaging. Serial imaging during the first week of life may provide a better understanding of the trajectory of the measured values and how they differ from those of healthy infants.
Demonstrating that hypothermia treatment of encephalopathic neonates correlates with normal ADC values and 1H-MR spectroscopy ratios reassures the clinician that injury to the cerebral microstructure and metabolism has been ameliorated. While we did not evaluate the association between DTI and 1H-MR spectroscopy with outcome, having normal conventional imaging and normal diffusion or metabolic parameters after therapy is important preliminary and prognostic information. We plan to correlate with medium-term (4-year) outcome when the children in our study reach that age.
Conclusions
The hypothesis that therapeutic hypothermia may ameliorate typical brain changes that follow hypoxia-ischemia, possibly by modifying the cascade of events that cause energy failure and cytotoxic edema, as evident from animal studies, is substantiated by the data presented here. In addition, DTI and 1H-MR spectroscopy, especially when combined, may be useful early biomarkers of treatment response during the first weeks of life following hypoxia-ischemia. Long-term follow-up studies are required to confirm these findings and determine the association between microstructure and metabolism following neuroprotective therapy and outcome.
Acknowledgments
We acknowledge the patients and their families who participated in this study and the nurses from the Neonatal Clinical Research Center for their dedication to our study and commitment to imaging neonates safely.
Footnotes
S.L. Bonifacio and A. Saporta contributed equally to this work.
This publication was supported by NIH/NCRR UCSF-CTSI grant number UL1 RR024131 and NIH grant P50 NS035902-12. Information on the National Center for Research Resources is available at http://www.ncrr.nih.gov/. Information on Re-Engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
The content of this work is solely the responsibility of the authors and does not necessarily represent the official view of NCRR or NIH.
Disclosures: Sonia Bonifacio—RELATED: Grant: NIH.* Hannah Glass—RELATED: Grant/Support for Travel to Meetings for the Study or Other Purposes: NINDS,* Comments: salary and travel to meetings supported by NINDS K23NS066137, UNRELATED: Payment for Lectures (including service on Speakers Bureaus): Child Neurology Grand Rounds, Cincinnati Children's Hospital Invited Speaker; First Neonatal Neurology Symposium, Riyadh, Saudi Arabia. David Glidden—RELATED: Grant: NIH.* Donna Ferriero—UNRELATED: Grants/Grants Pending: NIH,* Royalties: Elsevier. Anthony Barkovich—RELATED: Grant: NIH,* Comments: This is a grant to study brain injury in encephalopathic neonates, Support for Travel to Meetings for the Study or Other Purposes: NIH, Comments: reimbursed me for coming to meetings for the National Institute for Child Health and Development Vision, which was to come up with a research direction for the National Institute for Child Health and Development for the next 10 years, UNRELATED: Grants/Grants Pending: NIH. Duan Xu—RELATED: Grant: NIH R01EB009756.* *Money paid to the institution.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received October 7, 2011.
- Accepted after revision February 28, 2012.
- © 2012 by American Journal of Neuroradiology