Abstract
BACKGROUND AND PURPOSE: More than half of patients with TIA/minor stroke have ischemic lesions on early DWI, which represent irreversibly damaged tissue. The presence and volume of DWI lesions predict early deterioration in this population. We aimed to study the rate and implications of DWI reversal in patients with TIA/minor stroke.
MATERIALS AND METHODS: Patients with TIA/minor stroke were prospectively enrolled and imaged within 24 hours of onset. Patients were followed for 3 months with repeat MR imaging either at day 30 or 90. Baseline DWI/PWI and follow-up FLAIR final infarct volumes were measured.
RESULTS: Of 418 patients included, 55.5% had DWI and 37% had PWI (time-to-peak of the impulse response ≥2 seconds' delay) lesions at baseline. The median time from symptom onset to baseline and follow-up imaging was 13.4 (interquartile range, 12.7) and 78.73 hours (interquartile range, 60.2), respectively. DWI reversal occurred in 5.7% of patients. The median DWI lesion volume was significantly smaller in those with reversal (0.26 mL, interquartile range = 0.58 mL) compared with those without (1.29 mL, interquartile range = 3.6 mL, P = .002); 72.7% of DWI reversal occurred in cortically based lesions. Concurrent tissue hypoperfusion (time-to-peak of the impulse response ≥2 seconds) was seen in 36.4% of those with DWI reversal versus 62.4% without (P = .08). DWI reversal occurred in 3.3% of patients with penumbral patterns (time-to-peak of the impulse response ≥6 seconds − DWI) > 0 and in 6.8% of those without penumbral patterns (P = .3). The severity of hypoperfusion, defined as greater prolongation of time-to-peak of the impulse response (≥2, ≥4, ≥6, ≥8 seconds), did not affect the likelihood of DWI reversal (linear trend, P = .147). No patient with DWI reversal had an mRS score of ≥2 at 90 days versus 18.2% of those without reversal (P = .02).
CONCLUSIONS: DWI reversal is uncommon in patients with TIA/minor stroke and is more likely to occur in those with smaller baseline lesions. DWI reversal should not have a significant effect on the accuracy of penumbra definition.
ABBREVIATIONS:
- CATCH
- CT and MR Imaging in the Triage of TIA and Minor Cerebrovascular Events to Identify High-Risk Patients
- DEFUSE
- Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution
- EPITHET
- Echo-Planar Imaging Thrombolytic Evaluation Trial
- IQR
- interquartile range
- Tmax
- time-to-peak of the impulse response
- VISION
- Vascular Imaging of Acute Stroke for Identifying Predictors of Clinical Outcome and Recurrent Ischemic Events
Multiple studies have shown that more than half of patients with TIA/minor stroke have evidence of acute ischemic tissue injury on early DWI.1⇓–3 The presence and the volume of DWI lesions carry a negative prognostic value in this population.4⇓–6 The DWI-restricted lesions are thought to represent the irreversibly damaged ischemic core.7 This premise was recently brought into question by studies suggesting a high rate of DWI lesion reversal in patients with stroke who had undergone thrombolytic therapy.8,9 A recent systematic review of the published literature on DWI hyperintense tissue outcome reported variable rates of DWI reversal (0%–83%), with a mean reversal rate of 24% in patients with ischemic stroke.10 In most patients, the size of the acute infarct correlated with both the final infarct volume on follow-up T2/FLAIR imaging and the clinical outcome.11,12 Most previous work on DWI reversal has been undertaken in patients with moderate-to-severe strokes. Patients with TIA or minor stroke have smaller volumes of ischemia and potentially may have a higher likelihood of reversal. Previous imaging studies have reported reversal of the DWI signal in patients with TIA, but these were relatively small series, without scheduled follow-up imaging and DWI reversal was not studied systematically.13⇓–15
Potentially salvageable tissue known as the “ischemic penumbra” represents viable tissue at risk of infarct that has not yet infarcted.16 Various methods are used to define the ischemic penumbra on MR imaging, including the mismatch between perfusion and diffusion17 or clinical-diffusion mismatch.18 All of these definitions rely on DWI lesions representing irreversibly damaged ischemic core.
DWI reversibility, therefore, has implications in both accurate assessment of ischemic core and penumbra and outcome prediction.
We, therefore, aimed to determine the rate and characteristics of DWI reversal in 2 large prospective imaging cohorts of patients with TIA/minor stroke. We studied the correlation among the DWI lesion volume, lesion location, concurrent baseline hypoperfusion on perfusion-weighted imaging, the severity of the perfusion deficit, and the reversal of DWI signal on follow-up FLAIR/T2 imaging in this population.
Materials and Methods
Patients
Patients with high-risk TIA (focal weakness or speech disturbance lasting ≥ 5 minutes) or minor ischemic stroke (with initial NIHSS scores of ≤ 3) were prospectively enrolled. All patients presented to the Foothills Medical Centre, and informed consent was obtained before enrollment. The first cohort included patients with TIA/minor stroke who underwent perfusion MR imaging in the Vascular Imaging of Acute Stroke for Identifying Predictors of Clinical Outcome and Recurrent Ischemic Events (VISION) study.19 To be included in this substudy, patients had to be 18 years of age or older, have a premorbid mRs score of <2, have a CT scan of the brain and be examined by a stroke neurologist within 12 hours of onset, and have a brain MR imaging, including PWI, within 24 hours of onset. Patients were excluded if they had evidence of intracerebral hemorrhage or other nonvascular pathology on CT. The second cohort included patients who underwent PWI in the CT and MR Imaging in the Triage of TIA and Minor Cerebrovascular Events to Identify High-Risk Patients (CATCH)20 study. The inclusion criteria in CATCH were similar to those of VISION, with the exception that patients were included if they were assessed by a stroke neurologist and imaged within 24 hours of symptom onset. Standard clinical and demographic information was recorded for all patients. In both studies, secondary stroke prevention measures were implemented in accordance with current practice guidelines.21
MR Imaging Protocol
Patients in both studies had MR imaging brain scans completed as soon as possible and within 24 hours of symptom onset. Patients were imaged by using a 3T scanner (Signa VH/i; GE Healthcare, Milwaukee, Wisconsin) with an 8-channel head and a neck coil (Neurovascular Array Coil; Medrad, Indianola, Pennsylvania). Sequences included sagittal T1, axial T2, and axial FLAIR. Acute ischemic lesions were identified on the DWI sequence in the first cohort and on diffusion tensor imaging in the second cohort. DWI was performed with single-shot spin-echo diffusion echo-planar imaging with the following parameters: 240-mm FOV; twenty-seven 5-mm axial sections with 0.0-mm gap; a b-value of 1000 s/mm2 along 3 orthogonal directions; TR/TE, 9000/85–90 ms; acceleration factor, R = 2; matrix size, 192 × 192, zero-filled to 256 × 256, after April 2004. Before this date, the DWI sequences had the same parameters except that sections were 5-mm-thick and had 2-mm gaps. Parameters identical to those of DWI (after April 2004) were used in DTI imaging with 11 diffusion directions. Dynamic susceptibility-weighted contrast PWI was acquired by using a gadopentetate dimeglumine (Magnevist, 0.1 mmol/kg; Schering, Berlin, Germany) injection delivered via a power injector at 5 mL/s through an 18-ga needle in an antecubital vein, followed by a 20-mL saline flush at the same rate; and echo-planar gradient-echo (T2*) images were acquired every 2 seconds for 80 seconds (17 axial 5 + 0.0 mm gap sections at each time point).
Follow-up MR imaging without PWI was performed at day 30 in VISION and at day 90 in CATCH, respectively. The FLAIR sequence (TR/TE/TI, 9000/140/2250 ms; NEX, 1; FOV, 24 × 24 cm2; section thickness, 3.0 mm with no gap; acquisition time, 6 minutes 26 seconds) was used in all CATCH follow-up patients and in the VISION study after April 2004. Before April 2004, the follow-up FLAIR sequences (within VISION) had identical parameters except that the sections were 5-mm thick and there was a 2.0-mm gap between adjacent sections.
Image Analysis
MR imaging sequences (DWI/ADC, FLAIR, and T2) were reviewed for the presence of ischemic lesions at each time point. PWI source images (T2*) were imported into custom Matlab 7.4 (MathWorks, Natick, Massachusetts) software (PGUI Perfusion Analysis Software; Center of Functionally Integrative Neuroscience, Aarhus University Hospital Norrebrogade, Denmark; 2007).22,23 A whole-brain mask was drawn to include all cerebral regions and vessels within scanning range. An arterial input function was manually selected from the middle cerebral artery contralateral to the visible DWI lesion, and a block circulant deconvolution algorithm was used to calculate voxelwise maps of time-to-peak of the impulse response (Tmax).24 Maps of Tmax were imported into the Analyze software package (Biomedical Imaging Resource, Rochester, New York).25 Hypoperfused brain tissue was defined as those voxels with a Tmax delay of ≥2 seconds. A penumbral pattern was defined as the presence of PWI-DWI mismatch, where DWI lesion volume subtracted from the volume of lesions with a Tmax of ≥6 seconds' delay was greater than zero. Planimetric PWI and DWI lesion volume measurement was performed by using Quantomo software (Cybertrial, Calgary, Alberta, Canada).26
The intensity of hypoperfusion was characterized by the hypoperfusion intensity ratio, defined as the volume of tissue with severe hypoperfusion (Tmax ≥ 8 seconds) divided by the volume of tissue with any hypoperfusion (Tmax ≥ 2 seconds) as previously described.27 DWI hyperintense lesion borders were defined by using a semiautomated threshold intensity technique. We referenced these lesions to the corresponding areas on the apparent diffusion coefficient maps to avoid selecting regions of T2 shinethrough. The b = 1000 image was used as the primary template because quantitative ADC thresholds tend to vary depending on the time after stroke onset and concurrent perfusion status.28 For DWI volume measurement, a standardized display method with B0 images, was used to standardize the window width and level before volumetric measurements.29 The final infarct volume was measured on follow-up FLAIR sequences, with reference to the baseline FLAIR and DWI sequences.
All images were reviewed by 2 investigators (N.A. and J.M.) for persistence of T2 hyperintense signal or complete reversal of DWI signal on follow-up FLAIR imaging. In those who had apparent DWI reversal (ie, complete signal resolution), automated coregistration of the follow-up FLAIR images to the acute DWI was performed by a third reviewer (B.C.V.C.) using open-source McConnell Brain Imaging Centre Tools software (Montreal Neurological Institute, Montreal, Quebec, Canada) and visually verifying them for accuracy.
Primary and Secondary Outcome
The primary outcome was the rate of DWI reversal on follow-up FLAIR imaging. This was a priori defined as the absence of T2/FLAIR hyperintense signal on follow-up imaging in the region of the brain with the baseline DWI restriction. The patients were not considered to have DWI reversal if there was evidence of atrophy or gliosis in the same region that had DWI restriction or if they showed partial resolution of signal on follow-up imaging (Fig 1). Secondary outcome was the rate of clinical disability as defined by an mRS score of ≥2 at 90-day clinical follow-up.
Example of focal atrophy on follow-up FLAIR imaging in the region of the original DWI lesion that could mimic DWI reversal. Baseline DWI lesion (A) and baseline FLAIR (B) show the acute ischemic infarct. C, An area of focal atrophy in the region of the baseline DWI lesion.
Statistical Analysis
Statistical analyses were performed by using the Statistical Package for Social Sciences, Version 20.0 (IBM, Armonk, New York). Data are reported by using standard descriptive statistics. Parametric and nonparametric testing was applied where appropriate to assess clinical and imaging predictors of DWI lesion reversal.
Results
Baseline Clinical and Imaging Characteristics
A total of 418 patients in the pooled VISION (n = 137) and CATCH (n = 281) studies had DWI and PWI at baseline. The median age in the pooled cohort was 68.2 years (IQR = 21.3), and the median NIHSS score was 1 (IQR = 2); 45.5% had a history of hypertension; 13%, diabetes; 9.6%, known atrial fibrillation; and 16.3% were smokers at the time of the presenting symptoms. There were more male patients in VISION (60%) relative to CATCH (42%, P = .001); otherwise patients had comparable vascular risk factors in the 2 cohorts. The median time between symptom onset and baseline MR imaging was 13.4 hours (IQR = 12.7). At baseline, DWI positivity was seen in 55.5% (232/418; 95% CI, 50.6–60.3) of patients, and 37.3% (156/418; 95% CI, 32.8–42) had a perfusion deficit (Tmax ≥ 2 seconds delay). A total of 143/418 (34.2%; 95% CI, 29.7–39) patients had concurrent perfusion and diffusion deficits. The median volume of DWI-positive lesions was 1.15 mL (IQR = 3.36).
Follow-Up Imaging and DWI Reversal
Follow-up imaging was available in 80.6% (337/418; 95% CI, 76.5–84) of patients, of whom 57% (192/337; 95% CI, 51.5–62) had positive DWI lesions at baseline. Reasons for lack of follow-up imaging included a new contraindication to MR imaging (pacemaker insertion, mechanical heart valve insertion), death, or patient refusal.
The median time from symptom onset to follow-up imaging was 78.7 days (IQR = 60.2). In patients with positive DWI lesions at baseline, DWI reversal on follow-up imaging was seen in 5.7% (11/192; 95% CI, 3.2–9.9) of patients (Fig 2). The rate of DWI reversal was similar between the first and second cohorts (6%; 95% CI, 1.8–13.3) versus (5.6; 95% CI, 2.4–10.5), P = .91, respectively.
Example of a patient with DWI reversal. Baseline DWI (A) and baseline FLAIR (B) show the acute ischemic infarct corresponding to the patient's presenting symptoms. C, Complete resolution of the DWI signal on follow-up MR imaging (FLAIR).
The Table summarizes the clinical and radiographic characteristics of patients with DWI reversal relative to those without. More patients with persistent DWI lesions had concurrent perfusion deficits (tissue with Tmax of ≥2 seconds' delay) or concurrent ischemic penumbra ([Tmax ≥ 6 seconds]-DWI), though these differences did not reach statistical significance (Table). The likelihood of DWI reversal did not decrease with an increased severity of hypoperfusion, defined as greater prolongation of Tmax (≥2, ≥4, ≥6, ≥8 seconds' delay) (linear trend, P = .147). The hypoperfusion intensity ratio was not a predictor of DWI reversal in a regression analysis, adjusting for the volume of baseline DWI lesions (OR = 0.73 [95% CI, 0.01–36], P = .87).
Clinical and radiographic characteristics of the DWI-positive patients with or without reversal
A total of 22% of patients (92/418, 18%–26%) had evidence of ischemic penumbra (Tmax ≥ 6 seconds − DWI) > 0. The median volume of penumbra in these patients was 12.5 mL (IQR = 31.3, geometric mean = 9.63). When we adjusted for the cases with DWI reversal, the volume of baseline penumbra changed in only 3 patients. Moreover, 2 additional patients would have had penumbra at baseline when considering DWI reversibility. Despite this, the median baseline penumbra was not significantly changed after adjusting for the DWI reversibility (12.2, IQR = 30.3, geometric mean = 9.6).
The median DWI lesion volume was significantly smaller in those with reversal (median = 0.26 mL, geometric mean = 0.32 mL, IQR = 0.58 mL) versus those who did not reverse (1.29 mL, geometric mean = 1.47 mL, IQR = 3.6 mL, P = .002) (Table). Despite this, DWI reversal was not exclusively seen in patients with small baseline lesions. The largest DWI lesion with complete reversal on follow-up FLAIR measured 12 mL at baseline (Fig 2). Similarly, 22.6% (39/172; 95% CI, 16.6–29.7) of patients who did not show DWI reversal had baseline infarct volumes of <0.5 mL. The optimal volume for prediction of DWI reversal in this cohort was <0.4 mL, which correctly predicted infarct reversal with 80% sensitivity and 73% specificity (area under the curve = 0.77; 95% CI, 0.61–0.94; P = .002).
Correlation between DWI Reversal, Lesion Location, and Clinical Outcome
The Table shows the distribution of baseline DWI lesions in patients with or without DWI reversal. Most patients with DWI reversal had a small cortically based lesion (72.7%, 8/11; 95% CI, 45.1–91.7).
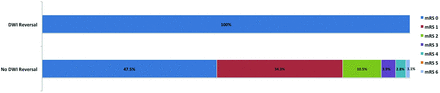
A total of 13.4% (56/418) of patients had a 90-day mRS ≥ 2. Figure 3 shows the distribution of mRS at 90 days in patients with follow-up imaging based on DWI persistence or reversal. Patients with DWI reversal were significantly less likely to be dependent (mRS > 2; 0%, 0/11) compared with those with persistent lesions (18.2%, 33/181, P = .028). In a multivariate regression analysis adjusting for age, infarct size, and DWI reversal, the only independent predictor of good outcome was infarct size (OR = 1.05; 95% CI. 1.01–1.1; P = .01).
The distribution of the modified Rankin Scale at 90 days in patients with follow-up imaging based on DWI persistence or reversal.
Discussion
This is the largest prospective perfusion-diffusion MR imaging study to date that addresses the clinical and radiographic characteristics of patients with TIA/minor stroke with DWI reversal. We found that complete DWI reversal is uncommon in this population and only occurs in 5.7% of patients. Patients with DWI reversal commonly had a small cortically based lesion. More patients with DWI reversal had early reperfusion, evident by the absence of concurrent tissue hypoperfusion on PWI. In our cohort, only 2 patients with evidence of ischemic penumbra had DWI reversal on follow-up imaging. Adjusting for the DWI reversal did not have a major impact on the calculated volume of baseline ischemic penumbra.
Our findings are in keeping with previous reports in patients with moderate and severe ischemic stroke in whom DWI reversal occurred on prompt recanalization with intra-arterial thrombolysis.8 Similarly, in a preliminary analysis of the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) data, diffusion reversal rates were significantly increased among patients in whom early recanalization was achieved and specifically occurred in the regions with normal baseline perfusion.30 In our cohort, although there was an association between tissue hypoperfusion and persistence of DWI lesion, this difference did not reach statistical significance. This discrepancy may be related to a relatively small total number of patients with reversal rather than a lack of association between reperfusion and DWI reversibility.
Our findings are in keeping with a pooled analysis of DEFUSE and Echo-Planar Imaging Thrombolytic Evaluation Trial (EPITHET) images, which reported a small median DWI reversal volume of only 1.5 mL.31 The previously reported higher rate of apparent DWI reversal in these cohorts was subsequently thought to be secondary to coregistration inaccuracies and infarct shrinkage related to gliosis/atrophy rather than true signal reversal. A separate analysis of the EPITHET data by Chemmanam et al32 reported a correlation between DWI reversibility and the severity of tissue hypoperfusion. Although our findings are in agreement with these results, we were unable to show a decline in the rate of DWI reversal with greater prolongation of Tmax. This discrepancy may, in part, be related to the very small median volume of infarct in our patients and complete (not partial) signal resolution used as the definition of DWI reversal in our cohort.
The pathophysiologic processes that result in the development of high signal intensity on DWI and corresponding low signal on the ADC maps are important in understanding the meaning and significance of acute DWI-restricted lesions. Putative mechanisms for development of DWI-restricted lesions include failure of adenosine triphosphatase–dependent Na+-K+ pumps, resulting in restriction of intracellular water motion.33 Others have suggested a reduction in the extracellular water content34 or alteration of pH due to anaerobic metabolism in the areas where adenosine triphosphatase levels are still maintained35 as potentially reversible causes of signal changes detected by diffusion-weighted imaging. The latter mechanisms suggest that DWI-restricted lesions can represent both areas of ischemic core and penumbra and may provide an explanation for its reversibility.
Our study has some limitations: Neither of the 2 cohorts included consecutive patients because this analysis was restricted to patients with TIA/minor stroke in whom we were able to obtain MR imaging scans within the first 24 hours of onset. Furthermore, it is possible that some early DWI reversals occurred before the initial MR imaging study was completed. Forty patients with a baseline DWI-restricted lesion did not have follow-up imaging, which can affect our reported rate of DWI reversal. Also, the follow-up imaging of 2 patients with DWI reversal contained 2.0-mm gaps; and as such, we cannot rule out the possibility that the apparent reversal may be related to technical issues in these 2 patients. Although 3 investigators separately assessed the images, the decision for DWI reversal was made on the basis of a consensus among the 3, and we did not perform inter-rater reliability for DWI reversal. Despite these limitations, this is the largest prospective study to date to address the rate and characteristics of lesions with DWI reversal in the population of minor stroke and TIA. We think it is unlikely that these results are due to infarct atrophy or focal gliosis in those regions because we used coregistration techniques combined with careful visual inspection of the baseline and follow-up images to ensure that the loss of DWI signal on follow-up T2/FLAIR imaging represented true DWI reversal.
Recanalization therapies remain a controversial area in patients with TIA or minor stroke presentation.36 Currently, there is an ongoing clinical trial to assess the efficacy of thrombolysis in this population.37 The volume of perfusion-diffusion mismatch has been suggested as a surrogate marker for selection of patients suitable for revascularization therapies.38 This volume is dependent on the correct definition of infarct core. Our results show that the infarct core is well-represented by DWI imaging and reversal is uncommon, especially in those with concurrent hypoperfusion. Mismatch volume can reliably be used for selection of eligible patients in future thrombolysis trials in minor stroke.
Conclusions
In summary, the presence of DWI lesions in patients with minor or completely resolved neurologic symptoms not only confirms the diagnosis of an ischemic attack but also has prognostic implications for recurrence of stroke and the development of disability in this population. The prognostic value of DWI reversal was not previously known in this population. Our results confirm that DWI reversal is uncommon but is associated with a more favorable profile and outcome in patients with TIA/minor stroke. DWI reversal usually occurs in those without concurrent perfusion deficits and should not have a significant impact on the estimated volume of the ischemic penumbra.
Footnotes
Disclosures: Negar Asdaghi—RELATED: Grant: Supported by a fellowship from the Canadian Institutes for Health Research and a research allowance from the Vancouver General Hospital and University of British Columbia hospital foundation. Ken S. Butcher—UNRELATED: Payment for Lectures (including service on Speakers Bureaus): Boehringer Ingelheim, Bayer Canada, Comments: Speakers fees for NOACs (unrelated to the current manuscript). Mayank Goyal—UNRELATED: Consultancy: Covidien, ev3, Comments: for teaching engagements, for trial design, Grants/Grants Pending: Covidien,* ev3*, Comments: partly sponsoring the ESCAPE (Endovascular treatment for Small Core and Anterior circulation Proximal occlusion with Emphasis on minimizing CT to recanalization times) trial, Payment for Lectures (including service on Speakers Bureaus): Covidien, ev3, Comments: For teaching engagements, Stock/Stock Options: Calgary Scientific Inc, NoNO Inc. Shelagh B. Coutts—RELATED: Receives salary support from Alberta Innovates - Health Solutions and the Heart and Stroke Foundation of Canada Distinguished Clinician Scientist award, supported in partnership with the Canadian Institute of Health Research and Institute of Circulatory and Respiratory Health and AstraZeneca Canada Inc. The VISION study was supported by grant funding from the Canadian Institutes for Health Research (CIHR MOP-118096) and Heart and Stroke Foundation of Alberta, Northwest Territories and Nunavut. The CATCH study was funded by grant funding from the Canadian Institutes for Health Research (CIHR MOP - 89937) and a Pfizer Cardiovascular research award. The 3T MR Scanner in the Seaman Family MR Research Centre used in this study was partially funded by Canada Foundation for Innovation., UNRELATED: Grants/Grants Pending: Genome Canada,* Heart and Stroke Foundation of Canada,* Canadian Stroke Network,* Alberta Innovates,* Comments: grant funding. *Money paid to the institution.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received April 5, 2014.
- Accepted after revision July 15, 2014.
- © 2014 by American Journal of Neuroradiology