Abstract
BACKGROUND: The protocol for optimal antiplatelet therapy to prevent thromboembolic and hemorrhagic complications in patients with cerebral aneurysms using an endovascular approach is not clear.
PURPOSE: Our study analyzed the safety and efficacy of prophylactic tirofiban administration compared with oral antiplatelet drug therapy.
DATA SOURCES: We used the PubMed, EMBASE, MEDLINE, and Cochrane library data bases.
STUDY SELECTION: Our study consisted of all case series with >5 patients that reported treatment-related outcomes of patients undergoing endovascular procedures pretreated with tirofiban or oral antiplatelet drug therapy.
DATA ANALYSIS: Random effects or fixed effects meta-analysis was used to pool the cumulative rate of complications, perioperative mortality, and good clinical outcomes.
DATA SYNTHESIS: Fifteen studies with 1981 patients were registered. Thromboembolic complications were significantly lower in the tirofiban group (3.6%; 95% CI, 1.9%–5.8%) compared with the dual-antiplatelet therapy group (8.5%, 95% CI, 4.5%–13%; P = .04). Pretreatment with tirofiban did not remarkably increase the rate of hemorrhagic complications (3.5%; 95% CI, 1.8%–5.6%) compared with dual-antiplatelet therapy (5.1%; 95% CI, 2.6%–8.5%; P = .371). There was a trend toward lower perioperative mortality with tirofiban (0.8%; 95% CI, 0.2%–1.6%) compared with dual-antiplatelet therapy (1.2%; 95% CI, 0.7%–2.0%; P = .412). There was no significant difference in the safety and efficacy between the tirofiban bolus plus drip and drip alone.
LIMITATIONS: The limitations are selection and publication biases.
CONCLUSIONS: Prophylactic therapy with tirofiban resulted in significantly lower rates of thromboembolic complications with no increase in hemorrhagic events or mortality than the prophylactic use of dual-antiplatelet therapy.
ABBREVIATIONS:
- ASA
- acetylsalicylic acid
- DAPT
- dual-antiplatelet therapy
- GP IIb/IIIa
- glycoprotein IIb/IIIa
- MINORS
- methodological index for nonrandomized studies
- PRISMA
- Preferred Reporting Items for Systematic Review and Meta-Analysis
Endovascular treatments such as stent-assisted coiling and flow diversion have recently emerged as an effective option for intracranial aneurysms.1,2 In general, thrombosis and subsequent ischemic events are major sources of morbidity and mortality in stent-assisted coiling procedures. Therefore, the adequate prevention of thromboembolic events is of paramount importance during stent-assisted coiling and flow diversion of intracranial aneurysms. Antiplatelet therapy offers partial prevention of these events.3,4 Many clinicians perioperatively use dual oral antiplatelet medications, routinely aspirin and clopidogrel, to prevent associated thromboembolic complications.5,6 However, thromboembolic complications still occur in up to 7%–40% of patients treated with dual-antiplatelet therapy (DAPT), even with high on-treatment platelet reactivity.7 The reported frequency of symptomatic hemorrhage during aneurysm embolization also varies greatly due to the irreversible inhibition of platelet aggression.8,9
The potent glycoprotein IIb/IIIa (GP IIb/IIIa) inhibitor tirofiban is increasingly used in acute coronary syndromes.10 Tirofiban provides distinct advantages due to its pharmacodynamic characteristics, such as a short onset of action and a short half-life. Several studies have evaluated its prophylactic use in the endovascular treatment of intracranial aneurysms.11,12
Despite the largest experience of alternative antiplatelet therapy, there are no publicly available guidelines on the use of prophylactic antiplatelet medications, and no systematic reviews evaluated the proper administration of these medications. Our meta-analysis was the first to investigate whether tirofiban is a conceivable alternative to DAPT as a prophylactic therapy for thromboembolism during the endovascular treatment of intracranial aneurysms. We compared clinical outcomes in patients pretreated with tirofiban versus DAPT during intracranial aneurysm treatment. We also performed subgroup analyses to compare outcomes of patients treated with a loading dose plus drip of tirofiban versus drip alone and patients treated with aspirin plus clopidogrel and aspirin plus ticagrelor therapy. This information will guide the selection of safer antiplatelet administration for endovascular treatment of intracranial aneurysms.
MATERIALS and METHODS
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines.13
Literature Search
Two reviewers independently searched the PubMed, EMBASE, MEDLINE, and Cochrane library data bases in March 2020 without restrictions on publication date or language. Search terms included “tirofiban,” “antiplatelet drug prophylactic,” “antiplatelet drip premedication,” “DAPT,” “oral antiplatelet medication,” “pretreatment antiplatelet,” “preventative use of antiplatelet,” “preprocedure antiplatelet,” “preventative clopidogrel,” “intracranial aneurysm,” “cerebral aneurysm,” “brain aneurysm,” “anterior cerebral artery aneurysm,” “anterior communicating artery aneurysm,” “posterior communicating artery aneurysm,” “posterior cerebral artery aneurysm,” “basilar artery aneurysm,” “middle cerebral artery aneurysm,” and “Berry aneurysm.” Search terms were combined using the Boolean operators “AND” or “OR.” References cited in the relevant articles were also reviewed.
Inclusion and Exclusion Criteria
Studies meeting the following criteria were eligible for inclusion: 1) a series of >5 patients, 2) studies with preprocedural antiplatelet medications in stent-assisted coiling of intracranial aneurysms, and (3) available data on periprocedural complications. We excluded studies performed using administrative/insurance data bases, articles that were duplicate reports of an earlier trial, reviews, and case reports.
Data Extraction
Two of the authors independently extracted the following information from the final set of included studies: first author’s name, year of publication, original country, sample size, type of agents used for pretreatment, method of administration, duration of follow-up, perioperative thromboembolic events, perioperative hemorrhage, and perioperative mortality related to antiplatelet therapy. The corresponding author of each study was contacted to obtain any missing information if required. Perioperative complications were events that occurred within 30 days of the procedure. Thromboembolic complications referred to ischemic stroke, territorial infarction, or >6 lesions with positive findings on DWI seen on 24- to 48-hour or long-term follow-up imaging. Hemorrhagic complications included intracerebral hematoma, subdural hematoma, subarachnoid hemorrhage, parenchymal hematoma, and groin puncture complications. Good clinical outcomes were defined as an mRS score of ≤2 at long-term (≥3 months) follow-up or a Glasgow Outcome Score of ≥4 at discharge.
Quality Evaluation (Bias)
Two authors assessed the quality of each study using the methodological index for nonrandomized studies (MINORS).14 The included studies were scored as 0 if not reported, 1 if reported but inadequate, and 2 if reported and adequate. Discrepancies were resolved via discussion and consensus. Quality was determined on the basis of 12 MINORS items. The items were scored as 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). Noncomparative studies with >12 points and comparative studies with >16 points were considered high-quality. Noncomparative studies with 8–12 points and comparative studies with 12–16 points were deemed intermediate-quality, and noncomparative studies with <8 points and comparative studies with <12 points were considered low-quality. The Grading of Recommendations Assessment, Development and Evaluation approach was used to assess the overall quality of the evidence for each outcome.15
Statistical Analysis
The meta-analysis was performed using STATA, Version 14.0 (StataCorp). Most of the included studies were noncomparative. Therefore, we estimated the cumulative incidence (event rate) from each cohort and the 95% confidence interval for each outcome. Pooled event rates were assessed for heterogeneity using the χ2 and I2 tests.16 A fixed effects model was performed with I2 ≤ 50%. Otherwise, a random effects model was performed. The incidence rates of the different outcomes were compared between tirofiban cohorts and DAPT cohorts using an interaction test as described by Altman and Bland17 or the χ2 test. We also performed subgroup analyses to compare outcomes of patients treated with a loading dose plus drip of tirofiban versus drip alone and patients treated with aspirin plus clopidogrel and aspirin plus ticagrelor therapy. P < .05 was considered significant in all analyses.
RESULTS
Search Results
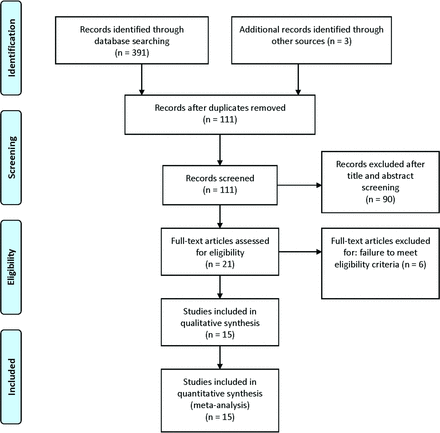
Three hundred ninety-one studies were retrieved from the PubMed, EMBASE, MEDLINE, and Cochrane library data bases. A total of 280 studies were excluded because they were duplicate items. Ninety studies were excluded after title and abstract screening. Six studies were removed because of the failure to meet the eligibility criteria; finally, 15 studies11,12,18⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-30 were selected for the meta-analysis. A flow diagram of the selection process for relevant articles is shown in Fig 1.
Flow diagram of the selection process for relative articles. A PRISMA–compliant search (www.prisma-statement.org) of MEDLINE, EMBASE, PubMed, the Cochrane library, and the Cochrane Central Register of Controlled Trials (CENTRAL) was performed.
Characteristics of Studies
The characteristics of the included studies are presented in the Online Supplemental Data. In total, 1981 patients were registered in the 15 included studies. A total of 613 patients were pretreated with tirofiban (30.9%), and 1368 patients (69.1%) received only dual oral antiplatelet medications. Among the patients who received tirofiban as a preventive measure in the stent-assisted coiling of intracranial aneurysms, 65 patients received drip alone and 548 patients received a loading dose plus a drip of tirofiban. Among the patients who received prophylactic dual oral antiplatelet therapy, 1278 patients received acetylsalicylic acid (ASA) plus clopidogrel, and 90 patients received ASA plus ticagrelor.
Quality Evaluation (Bias)
The assessment of the study-specific quality scores from the MINORS items is presented in the Online Supplemental Data. Items such as prospective collection of data, unbiased assessment of the study end points, and prospective calculation of the study size were not found in all 15 studies, and other items that were involved in most of the 15 studies were scored 2, which indicated good quality. Thirteen of the 15 studies were identified as of relatively intermediate-quality. Two of the 15 studies were considered low-quality.
Tirofiban versus DAPT
The results of the pooled event rates for each study are shown in Table 1. Thromboembolic complications were significantly lower in the tirofiban group (3.6%; 95% CI, 1.9%–5.8%) compared with the DAPT group (8.5%, 95% CI, 4.5%–13%; P = .04). Pretreatment with tirofiban did not increase the rate of hemorrhagic complications (3.5%; 95% CI, 1.8%–5.6%) compared with DAPT (5.1%; 95% CI, 2.6%–8.5%; P = .371). There was a trend toward lower perioperative mortality with tirofiban (0.8%; 95% CI, 0.2%–1.6%) compared with DAPT (1.2%; 95% CI, 0.7%–2.0%; P = .412). A rate analysis of good clinical outcomes indicated that the 2 approaches were associated with similar outcomes (89%; 95% CI, 74.0%–99.0%; and 92.7%; 95% CI, 87.1%–96.8%; P = .589) at follow-up or discharge.
Outcomes with tirofiban versus DAPT
Tirofiban Bolus plus Drip versus Drip Alone
The results of the pooled event rates for each study are shown in Table 2. There was no significant difference between the 2 approaches for the rates of thromboembolic complications (3.9%; 95% CI, 1.7%–6.8%; and 2.4%; 95% CI, 0.1%–7.5%; P = .513), hemorrhagic complications (3.0%; 95% CI, 1.8%–4.7%; and 2.5%; 95% CI, 0.1%–7.5%; P = .805), perioperative mortality (0.8%; 95% CI, 0.2%–1.7%; and 0.7%; 95% CI, 0.1%–4.1%; P = .927), or good clinical outcomes (86.3%; 95% CI, 62.8%–99%; and 94.7%; 95% CI, 86.6%–99.2%; P = .391). One study reported that after starting a drip protocol that no longer included a loading dose of tirofiban, the overall incidence of hemorrhagic complications fell impressively to 1.9% compared with 18.8% for the protocol that included a bolus dose plus a drip of tirofiban. No major or clinically significant bleeding events were observed using the drip protocol, even in patients with ruptured aneurysms.12
Outcomes with a bolus dose plus drip of tirofiban versus drip alone
ASA plus Clopidogrel versus ASA plus Ticagrelor
The results of the pooled event rates for each study are shown in Table 3. Patients pretreated with ASA and clopidogrel had a nonstatistically higher rate of thromboembolic complications (9.2%; 95% CI, 4.7%–14.8%) compared with patients pretreated with ASA and ticagrelor (5.4%; 95% CI, 1.7%–10.9%; P = .513). There was a trend toward a higher rate of hemorrhagic complications with ASA and clopidogrel (5.5%; 95% CI, 2.8%–9.1%) compared with ASA and ticagrelor (2.5%; 95% CI, 0%–10.5%; P = .321). For outcomes at long-term follow-up, perioperative mortality was nonstatistically lower in the clopidogrel group (1.2%; 95% CI, .6%–1.9%) than in the ticagrelor group (2.4%; 95% CI, 0.02%–8.5%; P = .583). The rate of good clinical outcomes was not significantly higher in the clopidogrel group (93.0%; 95% CI, 87.0%–97.2%) than in the ticagrelor group (93.1%; 95% CI, 84.3%–98.5%; P = .982).
Outcomes with ASA plus clopidogrel versus ASA plus ticagrelor
Meta-regression
The meta-regression model quantified the impact of the follow-up time on the P value of cumulative incidences (event rate). The results of the meta-regression analysis indicated that the follow-up period did not influence the effect estimate of tirofiban versus DAPT (Table 4) or ASA plus clopidogrel versus ASA plus ticagrelor (Table 5). The follow-up time of the drip group was not reported in original studies. Therefore, it was not possible to discern whether this factor influenced the heterogeneity in the tirofiban bolus plus drip versus the drip group.
Meta-regression analysis of follow-up time affecting heterogeneity (tirofiban versus DAPT)
Meta-regression analysis of follow-up time affecting heterogeneity (ASA plus clopidogrel versus ASA plus ticagrelor)
DISCUSSION
The main finding of our meta-analysis was that there was a significantly lower rate of thromboembolic complications (P < .05) from the prophylactic use of tirofiban than DAPT in patients with intracranial aneurysms undergoing endovascular treatment. There was no increase in intracranial hemorrhage in the tirofiban group (P > .05).
Stent-placement techniques and flow diverters are increasingly used in the management of intracranial aneurysms. Because thromboembolic events associated with stent placement and flow diverters occur often and these events correlate with poor clinical outcomes,31,32 antiplatelet agents (eg, prophylactic clopidogrel and aspirin) are used to prevent in-stent thrombosis and ischemic events.33 However, oral antiplatelet drugs take time to reach therapeutic levels. DAPT is routinely administered 3–5 days before endovascular treatment of intracranial aneurysms, and this therapy may be associated with a heightened risk (18.91%) of intracranial hemorrhage.34,35 GP IIb/IIIa inhibitors are considered the most powerful class of antiplatelet therapies, and their adjunctive beneficial effects were shown in several clinical trials.36⇓⇓-39
Tirofiban is a nonpeptide GP IIb/IIIa receptor, which is similar to abciximab because it has a high affinity for the GP IIb/IIIa receptor. However, tirofiban dissociates from the GP IIb/IIIa receptor more rapidly than abciximab. A relatively high level of platelet inhibition is achieved approximately 5–10 minutes after tirofiban administration, and inhibition of platelet function ≥95% at 10 minutes after the start of therapy is associated with a significant decrease in the incidence of major adverse cardiac events.40,41 Although the off-label use of tirofiban as a prophylactic and rescue treatment in neuroendovascular procedures is common, no systematic reviews have evaluated the safety and efficacy of tirofiban compared with DAPT as a prophylactic therapy during the endovascular treatment of intracranial aneurysms. The pooled data of our systematic review lend more support to the tirofiban strategy. Most of the studies included in this review indicated that the rates of thromboembolic complications from tirofiban and the DAPT strategy ranged between 0% and 10.0% and 1.7% and 26.5%, respectively. The rates of major hemorrhagic complications from the 2 therapies ranged between 0% and 10.5% and 0.9% and 10.2%, respectively, and higher rates were generally observed in studies with postoperative MR imaging. A study of 281 patients undergoing endovascular treatment of intracranial aneurysms22 revealed that thromboembolic events were observed more often in a DAPT group than a tirofiban group (10.8% versus 3.4%; P = .01), with no increase in the rate of intracranial hemorrhage (P = .16). Thromboembolic events in the ruptured subgroups were significantly fewer in the tirofiban subgroup than in the DAPT subgroup (3.9% versus 13.2%; P = .04), with no increase in the rate of hemorrhage (P = .36). Our study found significantly lower rates of thromboembolic complications in patients who received the tirofiban strategy for prophylactic treatment. There was a trend toward lower hemorrhage rates in patients who received the tirofiban strategy.
The short- and long-term clinical outcomes of the prophylactic strategy in the stent-assisted coiling of intracranial aneurysms are variable. The rates of perioperative mortality related to tirofiban and the DAPT strategy ranged between 0% and 3.4% and 0% and 2.0%, respectively. However, more included studies of the tirofiban strategy showed no death compared with the DAPT strategy (4 versus 1). The rates of good clinical outcomes in patients who received tirofiban and the DAPT strategy ranged between 70.0% and 97.7% and 87.8% and 97.8%, respectively. Zi-Liang et al22 reported no difference in good outcome (mRS 0, 1, and 2) at the 3-month follow-up in cases of thromboembolic events between the tirofiban and DAPT groups. The current meta-analysis observed that the prophylactic tirofiban strategy was associated with benefits in mortality (0.8 versus 1.2%), though this difference was not statistically significant because the studies were underpowered to detect a significant reduction in mortality. Our meta-analysis also showed no significant increase in mortality in patients who received tirofiban as a prophylactic therapy compared with DAPT. Overall, the tirofiban protocol provides a reasonable alternative to pretreatment with DAPT for flow-diversion and stent-assisted procedures.
The safety and efficacy of a bolus dose plus drip of tirofiban versus drip alone for prophylactic therapy are not well-established. Chalouhi et al12 examined a series of 67 patients undergoing stent-assisted coiling and found that a bolus followed by a maintenance dose of tirofiban appeared to have a high risk of cerebral hemorrhage. However, a maintenance infusion without an initial bolus had an exceedingly low risk of hemorrhage and appeared very safe and effective, even in the setting of subarachnoid hemorrhage. Although tirofiban is routinely used in percutaneous coronary interventions, few studies compared the safety and efficacy of different drug-delivery methods of tirofiban. Kirma et al42 performed a study of 47 patients undergoing percutaneous coronary intervention and demonstrated that microvascular perfusion, corrected Thrombolysis in Myocardial Infarction frame counts, myocardial blush grades, and long-term clinical outcomes did not differ significantly between patients with the intravenous bolus plus drip of tirofiban and an intracoronary bolus alone. Our meta-analysis found no significant difference between bolus plus drip and drip alone for the rates of thromboembolic complications, hemorrhagic complications, perioperative mortality, or good clinical outcomes.
The most commonly used first-line oral dual-antiplatelet regimen consists of aspirin and clopidogrel. Ticagrelor is an alternative in patients who are clopidogrel-resistant or clopidogrel-allergic. However, the safety and efficacy of aspirin plus clopidogrel versus aspirin plus ticagrelor for prophylactic therapy in stent-assisted coiling of intracranial aneurysms are also not well-established. The Platelet Inhibition and Patient Outcomes (PLATO) study found that the use of ticagrelor DAPT reduced the collective incidence of death due to myocardial infarction, stroke, and other vascular conditions compared with clopidogrel DAPT (9.8% versus 11.7%; hazard ratio, 0.84; P < .001).43 No statistically significant differences in the rate of major hemorrhagic incidents were found between patients administered aspirin plus ticagrelor or aspirin plus clopidogrel.43 However, the results of the POPular AGE (Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome)44 study showed that clopidogrel was a favorable alternative to ticagrelor in patients 70 years of age or older who presented with non-ST-elevation acute coronary syndrome because it led to fewer bleeding events without an increase in the combined end point of all-cause death, myocardial infarction, stroke, or bleeding. Clopidogrel may be an alternative P2Y12 inhibitor, especially in elderly patients with a higher bleeding risk.
Adeeb et al45 investigated 402 patients undergoing Pipeline Embolization Device (Medtronic) placement for the treatment of intracranial aneurysms and found that the risk of a high rate of thromboembolic complications was mitigated in nonresponders who were switched to the alternative ticagrelor. The current meta-analysis found no significant difference in the rate of thromboembolic complications, hemorrhagic complications, perioperative mortality, or good clinical outcomes between patients pretreated with aspirin plus clopidogrel and aspirin plus ticagrelor.
Limitations
The current meta-analysis has a number of limitations, including inevitable clinical heterogeneity among the included studies. There is also a significant publication bias for the variable patient selection and aneurysm features, which may affect the results. The evaluation of good clinical outcomes was inconsistent across studies, and mRS scores or Glasgow Outcome Scores were used in different analyzed studies for outcome assessment. Many included series were cases collected during several years, and it is possible that complication rates and mortality improved as a result of increased operator experience and skill and improved devices and technology. For the method of drug administration, there was variation in the timing of tirofiban and DAPT administration (just before procedures, before stent placement, before or after the deployment of flow diverter, just before procedure, at least 5 days before the procedures, and at least 7 days before the procedures). The comparisons reported in the current analysis were made across, not within, studies; this feature may greatly weaken the inferences. Most of the series included were noncomparative, and groups were not randomized. Finally, most of the tirofiban studies included in our analysis were limited to short-term mortality of <3 months, but GP IIb/IIIa inhibitor studies often demonstrated an increasing survival benefit with longer follow-up.46,47
CONCLUSIONS
The current meta-analysis revealed that the administration of tirofiban as a premedication had a significant benefit with favorable trends for lower rates of thromboembolic complications with no increase in hemorrhagic events compared with the prophylactic use of DAPT. No difference in outcome was seen on the basis of the method of tirofiban administration. Further evaluations in adequately powered large trials are needed to confirm the clinical benefit of this therapy.
Footnotes
This study was supported by the Clinical Practical New Technology Development Foundation of Qilu Hospital (2019-7).
Yanxiao Xiang and Hua Zhao contributed equally to this study.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.
- 36.
- 37.
- 38.
- 39.
- 40.
- 41.
- 42.
- 43.
- 44.
- 45.
- 46.
- 47.
- Received July 2, 2020.
- Accepted after revision November 2, 2020.
- © 2021 by American Journal of Neuroradiology