Abstract
BACKGROUND AND PURPOSE: To our knowledge, the upper limits of the thickness of normal meninges on neurosonograms are not known. We therefore established a nomogram for sonographic measurements of the leptomeninges (pia-glial plate) and assessed its usefulness in neurosonographic examinations of children with bacterial meningitis.
METHODS: The pia mater–cortical glia limitans complex on the surface of the brain and in the sulcus of a frontal gyrus was measured on neurosonograms in 100 infants without meningeal disease in order to establish a nomogram of the thickness of this pia-glial plate, referred to as the leptomeninx. Effects of prematurity, age, sex, and single-layer (surface) versus double-layer (sulcus) measurements were analyzed. Meningeal thicknesses derived from a retrospective analysis of the neurosonograms of 33 patients with purulent meningitis and a prospective study of 22 patients with bacterial meningitis were compared with the nomograms. Clinical outcomes of children with meningeal thickening were compared with those of affected children with normal meninges.
RESULTS: The distribution of sulci measurements was significantly asymmetrical around the mean. Statistical data showed no influence of prematurity and sex, but showed surface measurements to be more consistent than sulcal measurements. Older chronological age was related to slightly larger sulci, but did not influence the surface measurements. In children with bacterial meningitis, the surface meninges were less frequently thickened than were the sulci. Sulcal enlargement occurred often in combination with echogenic deposits in the subarachnoid space.
CONCLUSION: Leptomeninges are best measured on the surface of a gyrus rather than in a sulcus, as the normal thickness of the sulci shows much more variability. Clinical outcome of bacterial meningitis cannot be predicted by presence or absence of meningeal thickening as the only sonographic abnormality.
To our knowledge, the distribution characteristics of sonographic measurements of normal meninges (mean, SD, median, skew, and so on) have not been quantified, and meningeal thickening in meningitis has not been objectified. Leptomeningeal thickening has been reported in various pathologic conditions on transfontanellar sonograms (1, 2), CT scans (3), and MR images (4). Most sonographic descriptions are subjective, without measurements, and report such findings as a “tigerhead appearance” on coronal brain sonograms (5).
The purpose of this study was to establish a nomogram of leptomeningeal thickness in order to evaluate the meninges in pathologic conditions more objectively.
Bacterial meningitis is the most common cause of meningeal thickening in children. The diagnosis of meningitis is clinical (fever, bulging anterior fontanel, neck stiffness, irritability or lethargy, sometimes convulsions) and is proved by lumbar puncture and analysis and culture of CSF. Sonographic studies serve mostly to detect such complications as abscess, brain edema, hydrocephalus, and subdural effusions. Meningeal thickening has been described by various authors but has not been correlated with clinical status and outcome. Does documented meningeal thickening imply a poor prognosis?
The subarachnoid space is seen on transfontanellar sonograms in most infants. The arachnoid membrane, separated from the dura mater only by a virtual (subdural) space, is usually not distinguishable as an individual structure and cannot be separated from the echo signal arising from scalp, calvaria, dura mater, and arachnoid combined, unless there is a subdural effusion. The echogenicity of the brain surface thus reflects the echoes of the pia mater and the underlying glia limitans membrane of the cerebral cortex, to which it is closely attached. This pia-glial plate was measured on the surface of a frontal gyrus as a single layer and as a double layer inside a frontal sulcus.
Issues such as the best place to measure the leptomeninges and the influence of age, sex, and prematurity were addressed by statistical analysis.
Methods
One hundred infants aged 1 day to 64 weeks (median age, 8.5 weeks) without meningeal disease or intracranial hemorrhage exceeding grade 1 had transfontanellar sonography requested for various reasons, such as prematurity, apnea, convulsions, asphyxia, macrocrania, or to search for malformations in association with intestinal atresia or for malformative syndromes. Seventy-three were term infants; 55 were male and 45 were female. Premature infants of less than 28 weeks' gestation do not have sufficient gyral formation and therefore no sulci, and were excluded from the study. No sign of brain abnormality was seen in any of the infants on clinical follow-up examinations during hospitalization or upon their return to the hospital after discharge during the following year.
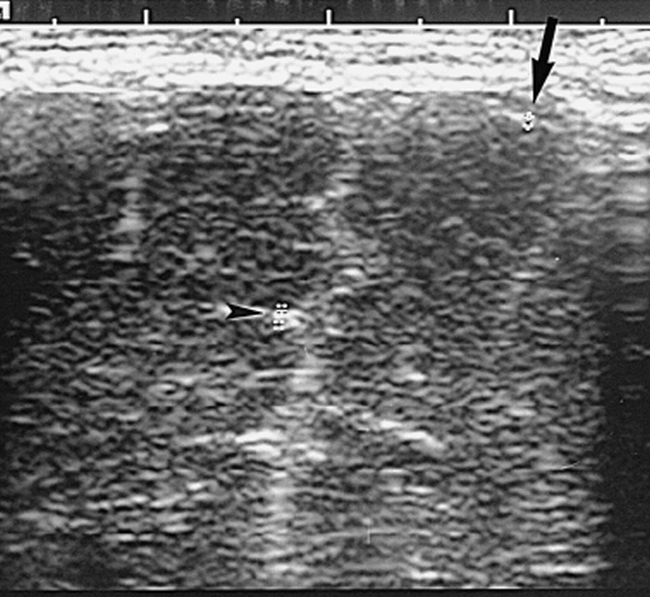
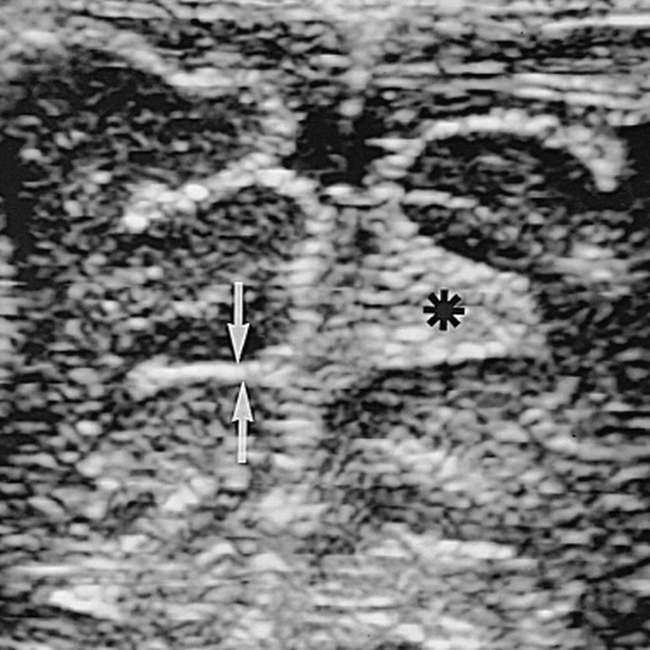
The neurosonograms were done with an Acuson sonographic machine (Mountain View, CA) with a 7- or 5-MHz sector scan head, depending on the size of the brain. In all infants, a 7-MHz linear transducer was used for examinations of the brain surface. The leptomeninges were measured on screen by electronic calipers on the surface of a gyrus (single measurement) and in a frontal sulcus (double measurement) on a coronal image (Fig 1) using the 7-MHz linear probe. Care was taken to make the measurement perfectly perpendicular to the meninges (as a slightly oblique plane would create an apparent increase in thickness, especially of a sulcus) and to measure the minimal sulcal width at the site at which the two investing surface layers began to touch but were neither fused nor compressed (as normal sulci may vary considerably in width from one to another and within the same sulcus) (Fig 2).
Coronal neurosonogram of a newborn term baby with duodenal atresia. The echogenic surface of a frontal gyrus (arrow) and the width of a sulcus (arrowhead) are measured
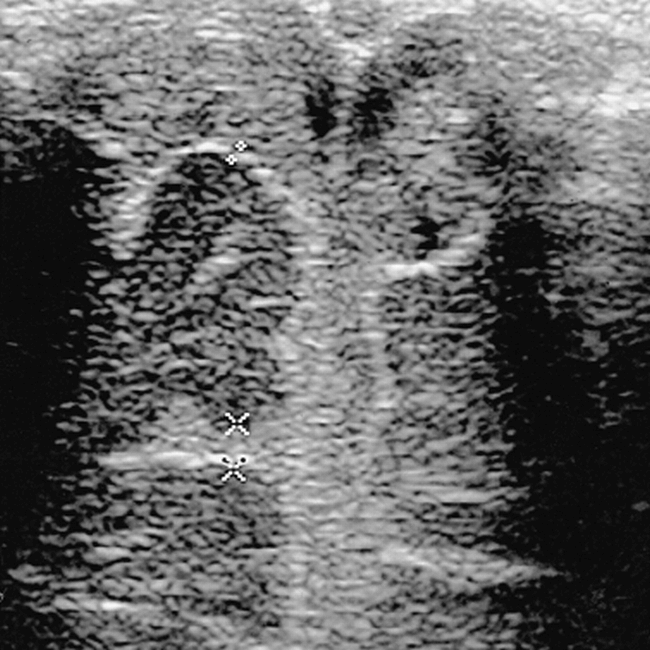
Coronal neurosonogram of a healthy 4-week-old baby shows a frontal sulcus of 2 mm on the left (arrows); however, on the right, a faulty measurement of 5.6 mm (asterisk) would have been obtained if the measurement criteria described in the text were not respected
Ninety-two percent of the sonographic studies were done by one of the authors, the others by a senior resident or fellow. The variables of age, sex, prematurity, and single and double measurements were recorded. Statistical analyses (Statistica, Statsoft, Tulsa, OK) were performed to determine the characteristics of distribution (eg, mean, SD, minimum, maximum, median, skew, and standard error of the skew of each measurement). The expected normal distribution around the mean was fitted on the histogram of the observed frequencies. Kolmogorov-Smirnov and Lilliefors tests were performed to assess the goodness-of-fit of a normal distribution with the histogram.
The effect of sex and prematurity was determined by analyses of variance (ANOVA) to assess the influence of these factors taken separately or as a function of their interaction. The regression of age in weeks with each measurement was computed for gestational age, chronological age, and the sum of both. The relationship between the two measurements was illustrated graphically as a scattergram with a polynomial regression of the third degree with its 95% confidence interval and also with an ellipse of the 95% confidence interval around the common means.
Clinical charts and the transfontanellar sonograms of 50 consecutive patients less than 1 year old with bacterial meningitis and neurosonograms obtained between 1988 and 1995 were reviewed retrospectively (group A). These patients represent about 75% of babies with purulent meningitis; in the other 25%, no neurosonogram had been requested by the clinician. Whenever feasible, the thickness of the meninges of the surface of a frontal gyrus and within a frontal sulcus was measured by hand with mechanical calipers. In 41 infants, the examinations had been done with a Diasonics (Milpitas, CA) sonographic machine using only a 7.5-MHz neonatal head transducer. The remaining nine patients were imaged with an Acuson machine and a 7- or 5-MHz sector scan, and seven of these nine had also been examined with a linear 7-MHz probe. After the nomogram was established, the meninges of 22 children with proved bacterial meningitis in whom sonograms had been obtained (group B) were measured prospectively at the same sites as in group A. In these patients, sonography had been performed using an Acuson machine with a 7- or 5-MHz sector probe as well as a 7-MHz linear probe. All measurements were done on the linear probe neurosonogram with electronic calipers. The clinical selection of the patients was similar in both groups.
The leptomeninges were considered to be thickened when a single layer was thicker than 1.3 mm and/or a sulcus was thicker than 2 mm. In both groups, the subarachnoid space was measured as the nearest distance between the brain surface and the ipsilateral wall of the sagittal sinus and was considered enlarged if it exceeded 3.2 mm (6). The echogenicity of the fluid in the subarachnoid space was considered normal when it was anechoic except for crossing vessels and abnormal when it contained echogenic particles or deposits. The final clinical outcome was compared with the results of the initial and, sometimes, follow-up sonographic examinations to assess the prognostic significance of meningeal thickening.
Results
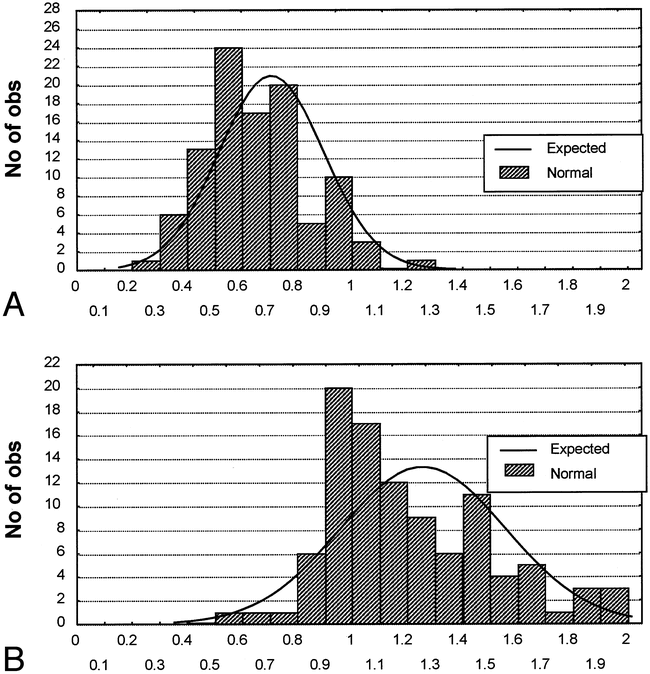
Table 1 shows the distribution characteristics of the measurements of the normal leptomeninges used for the nomogram. Surface meninges (single measurements) had a thickness (mean ± SD) of 0.71 ± 0.19 mm; range, 0.3 to 1.3 mm; median (50th percentile), 0.70 mm. The thickness of the sulci (double measurements) was 1.26 ± 0.30 mm; range, 0.6 to 2 mm; median, 1.20 mm. The mean, median, SD, and 95% confidence intervals of the mean of the double measurements (sulci) were almost twice those of the single measurements (surface). The skew characteristic provides the degree of asymmetry of the distribution (which is 0 for a normal distribution). The symmetry of distribution for the single measurements and the distribution of the double measurements were significantly skewed to the right (higher values); therefore, high readings in healthy infants could have been misinterpreted as pathologic findings. Lilliefors test, comparing the observed distribution (the histograms) with the statistically normal distribution around the mean, was significant, confirming the fact that the distribution of the measurements was not normal around the mean for single and double measurements.
Descriptive statistics of normal meningeal thickness (in mm)
To illustrate these findings, the histograms of single (Fig 3A) and double (Fig 3B) measurements are shown. The distribution of single measurements and the observed double measurements do not fit the expected values of a normal distribution. Indeed, lower readings are too high, and higher readings are also too high. The Kolmogorov-Smirnov test was significant: D = .15094, P < .05, and D = .15830, P < .05 for the single and double measurements, respectively. Lilliefors test was also significant (P < .01) for both measurements. These results illustrate the significant lack-of-fit of the expected normal distribution of the observed frequencies of each of the histograms. As shown in the scatter plot (Fig 4), an ellipse of 95% confidence limits around both means illustrates the relation between the two measurements. A number of double measurements greater than the expected 95% will not fit into the ellipse, owing to outliers, especially for higher values. This ellipse is another way to illustrate the combination of two normal distributions. Even if the correlation between the single and double measurements was significant (r = .435, P < .001), a great deal of dispersion is present around the polynomial regression line, owing to scattered sulci readings.
A and B, Histograms of observed distribution of the readings of single measurements (surface, A) and double measurements (sulci, B) with the expected normal distribution computed with the SD around the mean. The normal, symmetrical, expected distribution does not satisfactorily fit the observed data of the single and double measurements. Kolmogorov-Smirnov test: D = .15094 for single measurements, and D = .15830 for double measurements; P < .05 for both measurements. Lilliefors test: P < .01 for both measurements. All tests were significant, demonstrating an insufficient goodness-of-fit of the expected normal distribution with the observed frequencies
Scatterplot of single and double measurements, with third-degree polynomial regression and its 95% confidence limits and an ellipse of the 95% confidence limits around the common mean of both measurements. A number of readings are outside the 95% confidence limits. The scales of the measurements differ for graphic reasons. The correlation coefficient is statistically significant (r = .435, P < .001). Most outliers are located at the higher end of the measurements
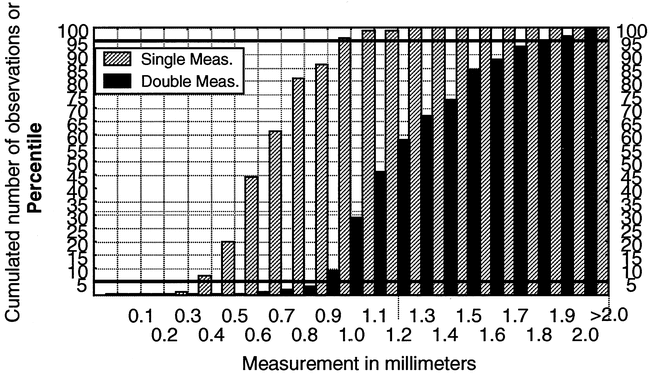
The significant lack-of-fit of the normal model, especially on the side of higher readings, which should represent the critical boundaries between normal and abnormal findings, requires the use of a nonparametric description of the data. The histograms of cumulated frequencies are shown in Figure 5, with the same scale for both measurements. Because 100 subjects were used, this represents the equivalent of a percentile distribution. The 95th percentile is graphically equal to 1.0 mm and 1.8 mm for the single and double measurements, respectively. Smoothing of the histograms can be done by hand by connecting the middle of the successive columns. Any value of decile, quartile, or median can be recovered directly on the chart, without any assumption of a mathematical underlying function, except for continuity.
Histogram of the cumulated frequencies of the surface and sulci measurements on the same scale. Because the number of subjects is 100, the cumulated frequencies are equivalent to percentile distribution. For illustrative purposes, the 5th and 95th percentiles are delineated. The median is equal to the 50th percentile. Smoothing of the graph can be done by hand by joining the center of each consecutive column of cumulated frequency
Some additional analyses were also performed for completeness. The ANOVA test (results not reported), performed separately on the single and double measurements, showed no significant effect of sex or prematurity. There was no significant regression of single-surface measurements with gestational age, chronological age, or the sum of both (measured in weeks). There was, however, a significant positive correlation between sulci measurements and chronological age (r = .357, P < .003) and between sulci measurements and the sum of gestational age and chronological age (r = .396, P < .001), but not between sulci measurements and gestational age alone.
An ancillary finding on the histograms was the unequal frequency of reporting for the different thickness readings in tenths of a millimeter. This suggests that the resolution of the measurements was closer to 0.2 mm than to 0.1 mm, although the reported axial resolution of the machines is 0.3 mm. This finding should not affect the distribution measurements.
The results of the neurosonograms of children with bacterial meningitis are summarized in Table 2. To reiterate, in group A, meningeal thickness was analyzed retrospectively and in group B, the analysis was prospective. Surface meninges were not measurable in nine (18%) of 50 patients in group A and in four (18%) of 22 patients in group B; the reasons included a faulty technique and a lack of examination with a liner scan head in two patients in group A, and compressed subarachnoid space and brain edema or dilated ventricles in the others. Sulci could not be measured in two (4%) of 50 children in group A and in four (18%) of 22 patients in group B, because of brain edema or hydrocephalus. The subarachnoid space was obliterated in 10 patients in group A and in three patients in group B, preventing measurement and assessment of the echogenicity of the fluid.
Sonographic findings (and clinical outcome) in infants with bacterial meningitis
Increased surface meningeal thickness was found in 15 (37%) of 41 children in group A and in five (28%) of 18 children in group B; all these patients also had enlarged sulci. Sulci were found to be thickened in 28 (58%) of 48 children in group A and in eight (44%) of 18 children in group B. Echogenic subarachnoid fluid was seen in 13 (33%) of 40 patients in group A and in eight (42%) of 19 in group B (Fig 6), and was seen in combination with sulcal enlargement in all but one patient with aseptic meningitis who had been treated with antibiotics before admission to the hospital. Ventricular enlargement was seen in 13 patients in group A and in two patients in group B. Only three needed drainage.
Coronal sonogram of a 3½-week-old infant with pneumococcal meningitis. The surface meninges are of normal thickness (0.8 mm). The subarachnoid space, the interhemispheric fissure, and the sulci are filled with echogenic material (pus and proteins by lumbar puncture). The frontal sulcus is enlarged (2.4 mm) and its undersurface is poorly defined
The clinical outcomes of the patients with meningitis were as follows: four children in group A and two in group B died soon after hospitalization (toxic shock, disseminated intravascular coagulation, cardiac arrest). In all but one of the children in group B, the surface meninges could not be measured because of brain edema, and, in one child in each group, the sulci were not measurable. One patient in group B, who had normal-appearing meninges, died rapidly of shock. Neurologic sequelae were seen in five patients in group A and in two in group B. Four of them had thickening of both the surface meninges and the sulci; in one, only the sulci were thickened. In one patient in group A, with later severe psychomotor retardation, neither the surface meninges nor the sulci were measurable. Only one infant with an infected ventriculoperitoneal shunt had normal-appearing meninges, and this patient was the only one in whom the abnormal neurologic status preceded the episode of meningitis. Clinical outcome was favorable in all other patients (41 in group A, 18 in group B), including 16 children with increased surface meningeal thickening and 31 with sulcal enlargement.
Discussion
The meninges originate from mesenchymal cells surrounding the early embryonic telencephalon. These cells separate into three layers: the outer layer forms the dura mater (and calvaria), the intermediate layer becomes the arachnoid (7). Neither of these can normally be identified by sonography, as they cannot be separated from the bony calvaria or the skull base. The inner pia mater, however, separated from the arachnoid by the fluid-filled subarachnoid space, is easily seen on sonograms in most patients with an open anterior fontanel. It closely follows the cortex of the brain and invests the sulci. Histologically, it contains collagenous fibers on the outside and elastic fibers on the inside, adheres to the neural tissue to form the pia-glial plate, and contains bridging vessels.
Meningeal thickening is nonspecific and may be due to an increase in any of the normal constituents, such as collagen deposition; fibromatosis (8); infiltration by inflammatory cells (eg, infection) (9), rheumatoid disease (10), sarcoidosis (11); vasculitis (12); infiltration by benign or malignant neoplastic cells, (eg, gliomatosis) (13), lymphoma (14), carcinoma (8), or melanosis (15); deposition of abnormal metabolic products (eg, Gaucher disease, amyloidosis) (16), hemosiderin deposit (17); or dysplastic angiomatosis (18) (Sturge Weber disease). Reactive collagen deposition can occur after surgery (19) (eg, after shunting of hydrocephalus). Reactive meningeal thickening is also seen after intrathecal chemotherapy (20). Many of these diseases may not cause macroscopic meningeal thickening; and although it seems useful to have reproducible criteria for the upper limits of normal meningeal thickness, normal values do not indicate absence of disease.
Sonography reveals not only the pia mater but also the underlying glia limitans. Many pathologic entities affect the superficial and penetrating vessels, the perivascular space, the blood-brain barrier, and therefore the pia-glial complex, which is seen as a unique echogenic structure. In establishing a nomogram for normal thickness of the leptomeninges by sonography, statistical computation is justified to determine the validity of the measurements, especially the boundary between health and disease, if the readings are to be interpreted by inference from a model of suitable distribution. The characteristics of distribution are of importance in determining whether the distribution is statistically normal. If normality is proved, mean, SD, and parametric comparison tests can be used (ie, t-test, ANOVA). If normality is not confirmed, nonparametric procedures must be used; for example, description in percentiles and nonparametric comparison tests (ie, Wilcoxon, Mann-Whitney).
The results of the various computations carried out to determine the characteristics of the distribution establish the usefulness of single measurements in determining acceptable upper limits in healthy infants. The same computations with double measurements provide evidence that great care should be exercised in the interpretation of high readings. Readings of both measurements will not add precision to the assessment of the clinical status of an infant, as shown by the great number of outliers off the 95% limits of the ellipse around the common means (Fig 4). The safe way to assess the meaning of a measurement is to use a percentile distribution, which does not imply any mathematical function except continuity. A much greater number of cases could justify the smoothing of individual columns.
The axial resolution of the linear transducer used in this study was 0.3 mm for the Acuson machine. These dimensions are close to those we wanted to measure. Use of higher frequency transducers may improve the accuracy of our data.
In children, meningitis is by far the most common cause of leptomeningeal thickening, followed by posthemorrhagic or postsurgical reaction. Transfontanellar sonography is often requested in newborns and infants with meningitis to look for such complications as hydrocephalus, abscess, ventriculitis, encephalitis, and so on. Meningeal thickening has been reported in many infants with purulent meningitis, but the prognostic value of such a sonographic finding has not been established.
In our series of patients with bacterial meningitis (groups A and B), meningeal thickening occurred in the sulci in 44% to 56% and in the surface leptomeninges in only 28% to 39%; 35% had echogenic subarachnoid fluid. All patients with echogenic subarachnoid fluid also had sulcal enlargement. We presume that increased meningeal thickness may be due to deposits of pus and fibrin as well as to actual infiltration by inflammatory cells. All patients in whom de novo neurologic sequelae developed had meningeal thickening at sonography, except for the one in whom the meninges could not be measured because of brain edema. However, over 75% of patients with similar sonographic findings of the meninges recovered clinically. Brain edema and compression of the meninges to a point where they could not be measured was related to an unfavorable outcome.
Conclusion
Meninges are considered to be abnormally thick when neurosonographic measurements exceed 1.3 mm on the surface of a gyrus or 2 mm within a sulcus. Absence of meningeal thickening does not exclude a diagnosis of meningitis, as brain edema may prevent visible meningeal thickening, and fatal toxic shock may occur very rapidly, leaving no time for the meninges to react in a visible way. Although the majority of the children with neurologic sequelae after meningitis also had meningeal thickening during the active phase of the disease, most of the children with bacterial meningitis and meningeal thickening did well. No clinical prognosis should be established on the basis of meningeal thickening as the only abnormal sonographic finding.
Footnotes
References
- Received July 31, 1998.
- Accepted after revision March 5, 1999.
- Copyright © American Society of Neuroradiology