Abstract
BACKGROUND AND PURPOSE: Landau-Kleffner syndrome (LKS) is epileptiform aphasia acquired during childhood and occurring in children with previously normal language development. The epileptiform activity in these children is thought to result in a functional ablation of eloquent speech areas. The purpose of this study was to investigate the usefulness of magnetoencephalography (MEG) for localizing the source of epileptiform activity in these patients.
METHODS: Nineteen patients with acquired aphasia and a suspected diagnosis of LKS were referred for MEG evaluation. Patients ranged in age from 4 to 14 years. Fourteen MEG studies were performed on a 74-channel system, four on a 148-channel whole-head system, and one on a 37-channel system.
RESULTS: Thirteen of the 19 patients had perisylvian MEG spikes. In 10 of the patients, the spikes were bilateral, and in three they were unilateral. Four other patients had non-sylvian spikes, and two patients had no spikes recorded. The results of MR imaging were normal or noncontributory for all 19 patients.
CONCLUSIONS: MEG can play a useful role in evaluating children with LKS and acquired epileptiform aphasia, both in diagnosis and in aiding presurgical localization of epileptiform activity when surgery is being considered.
In 1957, Landau and Kleffner (1) reported the cases of six children with developmentally normal language function who then developed aphasia in association with a convulsive disorder. This has since come to be termed the Landau-Kleffner syndrome (LKS). The peak age at time of onset is between 3 and 8 years (2). The language dysfunction may have an acute or insidiously progressive onset and may fluctuate in severity. Typically, a receptive disturbance, termed verbal auditory agnosia (3), is the initial dominant feature and often leads to mutism and complete unresponsiveness to sounds (4). Many of these children are incorrectly diagnosed as having acquired deafness unless EEG is performed (5).
All of the children have abnormal EEG results but all may not display seizure activity (2). The type of seizure may be simple partial motor, generalized tonic-clonic, atypical absences, or, rarely, myoclonic astatic (6). Paroxysmal EEG discharges may be generalized, bilateral, and multifocal. Temporal lobe predominance is found in 85% of the patients. In 15% of the patients, the discharges are reported to be unilateral and also show temporal lobe predominance (5, 7). More than 85% of the patients have been reported to have continuous spike and wave discharges during slow-wave stages of sleep (CSWS) (8). In many children, the degree of language dysfunction correlates with the severity of the EEG abnormality, as in the original report by Landau and Kleffner (1). In others, improvement or worsening aphasia occurs independently of the EEG abnormalities (4, 6). Anticonvulsant medications readily control clinical seizures, which usually resolve by 15 years of age. By contrast, variable degrees of language dysfunction persist into adulthood (4, 6).
The pathogenesis of this disorder is poorly understood. The results of MR and CT studies are typically normal. Identification of the primary source of epileptiform discharges is important both in understanding and in treating this disorder. Multiple subpial transections have recently been shown to be of value in treating the language disorder (4, 9). Precise localization of the primary source is difficult to identify with scalp-recorded EEG (6). Several recent reports have shown the usefulness of magnetoencephalography (MEG) in localizing epileptic activity (10−20). Magnetic source imaging (MSI) superimposes MEG localizations on the MR images and yields improved spatial resolution as compared with surface EEG. The purpose of this study was to report our experience with MEG in the cases of 19 patients who were referred with acquired aphasia and a suspected diagnosis of LKS.
Methods
Patients
Nineteen patients with acquired aphasia and a clinical diagnosis of suspected LKS were referred from multiple centers for MEG evaluation. Patient data are summarized in the Table. The patients ranged in age from 4 to 14 years. There were 12 male and seven female patients studied during a 3-year period. One patient was studied twice; examinations were performed 13 months apart.
SUMMARY OF MEG, EEG and MR studies in 19 children with LKS and acquired epileptic aphasia
MEG
Fourteen MEG studies were performed on a 74-channel dual probe Magnes II biomagnetometer (Biomagnetic Technologies Inc., San Diego, CA), four studies were performed on a 148-channel Magnes 2500 WH whole-head system (BTI), and one early study was performed on a 37-channel Magnes single-probe system. Each probe of the single- or dual-probe systems covered a recording area of approximately 15-cm diameter over the scalp. The single-probe system was suspended from a ceiling-mounted gantry, and the 74-channel system added a second probe mounted on a positioning device resting on the floor. The patients rested on an adjustable bed with the probes placed in contact with the head. Eight to 10 probe positions were typically used with the 37-channel system, and four symmetric probe positions, F3/F4, P3/P4, F7/ F8, and T5/T6, and one anteroposterior position, Fpz/Oz, were used with the 74-channel system. The whole-head system uses a helmet-shaped probe that is centered over the head with the patient lying supine and no repositioning of the probe is required.
Scalp EEG was recorded concurrently with the MEG, using an international 10–20 system, 20-channel bipolar montage (Neurofax 440 A; Nihon Kohden, Tokyo, Japan). The simultaneous EEG served to assist in spike identification, recorded real time, and avoidance of collection and analysis of data confounded by artifact. Electrocardiographic recording was also used to filter out the large magnetic signal generated by the heart, which can distort MEG recordings. Fiducial points at the nasion and left and right preauricular points along with the surface of the patients' scalp were digitized for subsequent data analysis and superimposition of the MEG source localizations on the MR images. MEG and EEG outputs were monitored on real-time displays by trained technologists. A trigger was activated to record an epoch of 5 s of preceding data and 1 s of postactivity data into the buffer memory, at a sample rate of 300 Hz and a band pass of 0.1 to 200 Hz, when epileptiform spike or sharp wave activity was observed. Data recordings typically lasted 2.0 to 2.5 hours.
MEG Analysis
MEG and EEG wave forms were digitally filtered at a band pass of 3 to 70 Hz. Visually selected spike or sharp wave activity was digitally marked and mapped by using a single equivalent current dipole model at each sampled time point. The model assumes a spherical head shape. The diameter and center of the sphere are chosen based on the previous head digitization. The equivalent current dipole model uses an iterative algorithm to calculate the location, strength, and orientation of the current dipole that best accounts for the actual magnetic field pattern measured. To be considered significant, the correlation of fit must be greater than or equal to 0.98 with the recorded field pattern and have a physiologically realistic current magnitude (Q < 400 nAm). The detected magnetic field must have a root mean square average of at least 400 femto-T, thus assuring an adequate signal-to-noise ratio. When a large number of dipole sources were detected, a clustering algorithm was used to concentrate the data in the regions with the most frequent spike activity.
MR imaging was performed on a 1.5-T imager (Signa; General Electric, Milwaukee, WI). Five-millimeter sagittal and axial T1-weighted images and 5-mm axial and coronal T2-weighted images were obtained. Fiducial markers were placed on the nasion and bilateral preauricular points during MR imaging. These coordinates were then used to map the MEG dipoles onto the MR images using interactive software (MR overlay, Biomagnetic Technologies, Inc.).
The patients were sedated during the MEG recordings and MR studies with IV administered propofol in 13 studies and orally administered amitriptyline in five studies (Table). In two studies, no sedation was used.
Results
The results of the MEG are summarized in the Table. MEG revealed perisylvian spikes in 13 of the 19 patients studied. These were bilateral in 10 patients (Fig 1) and unilateral in three (Fig 2). Of the patients with unilateral perisylvian spikes, one had left-sided spikes and two had right-sided spikes. One of the patients with right-sided spikes had a questionable diagnosis of LKS; the other had right posterior frontal and parietal perisylvian spikes.
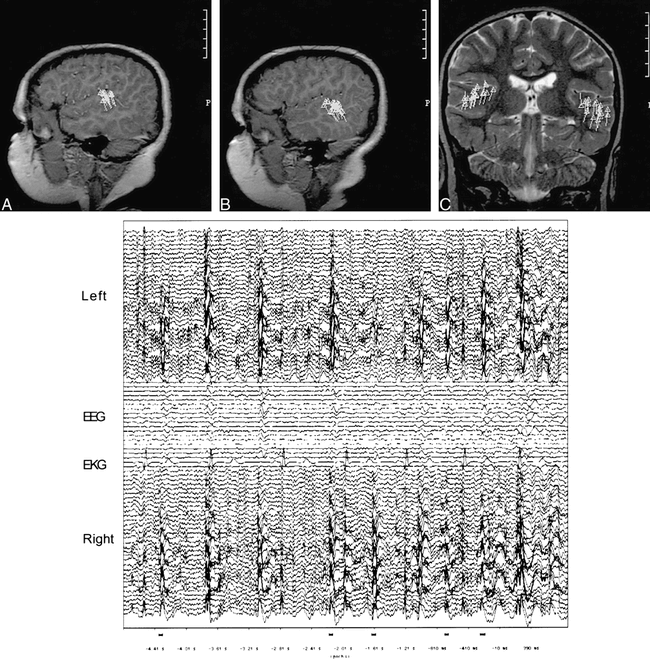
FIG 1. Images from the case of a 6-year-old male patient.
A, Sagittal T1-weighted image from the right hemisphere shows perisylvian clustering of spike activity in the posterosuperior temporal gyrus or Wernicke's area.
B, Sagittal T1-weighted image from the left hemisphere shows perisylvian clustering of spike activity in the posterosuperior temporal gyrus or Wernicke's area.
C, Coronal T2-weighted image shows bilateral perisylvian clustering of spike activity in the posterosuperior temporal gyrus or Wernicke's area.
D, Six-second data epoch shows MEG wave forms from the left hemisphere above, right hemisphere below, and concurrent EEG in the middle. Numerous spikes are present bilaterally.
FIG 2. Image from the case of a 10-year-old female patient. Sagittal T1-weighted image shows unilateral clustering of spike activity in the left posterotemporal lobe, with spikes bordering the posterior aspect of the left sylvian fissure
Four of the 19 patients had non-sylvian MEG spikes. One of these four had bilateral middle frontal gyrus spikes; one had left central and parietal spikes; one with questionable LKS had right parietal occipital spikes; one with a diagnosis of LKS versus autism had left middle frontal gyrus spikes.
Two of the 19 patients did not have MEG spikes. One of these patients was uncooperative during the recording session. The second patient had a questionable diagnosis of LKS.
One of the 19 patients was studied twice, with examinations performed 13 months apart (case 5a and b in the Table). The first MSI study showed bilateral perisylvian spikes. A second study performed after a right temporal lobectomy showed left frontal and left temporal perisylvian spikes. EEG studies performed concurrently with MEG agreed with the general region of MSI spike detection in 16 of 19 patients, but MSI was more precise in yielding more focal localizations.
Six of the 19 patients were reported to show continuous spike wave activity during CSWS, as revealed by previous EEG performed at their referring institutions. Among these six patients, MEG detected bilateral perisylvian spikes in three, bilateral midfrontal gyrus spikes in one, and occasional right parietal spikes in one. One patient was uncooperative during the MEG recording and had an incomplete study with no spikes detected. The results of MR imaging were essentially normal in 18 patients and showed right hippocampal atrophy in one.
Discussion
MSI localized epileptiform activity in 17 of the 19 children referred with acquired aphasia and a suspected diagnosis of LKS. The MSI localizations were more precise than EEG and were useful in confirming the diagnosis of LKS and in helping to understand the cause of the associated language dysfunction. Thirteen of the patients had spikes in a perisylvian location, thus supporting the concept first proposed in 1957 by Landau and Kleffner (1) that persistent epileptic discharges cause a functional ablation of areas concerned with linguistic communication. Morrell et al (4) further suggested that excessive epileptic discharge during the childhood period of language development results in functionally inappropriate synaptogenesis and then deficient synaptic pruning in the same regions subserving language function in children with LKS.
The cause of epileptiform activity in LKS is unknown. As in the cases of our patients, MR imaging results are either normal or noncontributory. Holmes et al (21), in contrast to the idea of epileptic activity causing aphasia, suggested that both the epileptic discharges and the aphasia were epiphenomena of underlying pathologic abnormalities in the speech areas. Deonna (6) reviewed the arguments both for and against epileptiform causality of aphasia. Supportive evidence included spectacular speech recovery in some patients, coincident with disappearance of EEG abnormalities when treated with steroid or antiepileptic agents, as well as the success of multiple subpial transections in alleviating language dysfunction. For patients with bilateral EEG spikes, intracarotid sodium Amytal injection or systemic methohexital injection has been used to confirm a unilateral hemisphere as the primary generator (4, 6). Arguments posed against an epileptic cause include the development of clinical seizures in some children long before or long after the onset of language dysfunction. Additionally, some children never develop clinical seizures, although they typically do have abnormal EEG results.
The reported EEG abnormalities in cases of LKS and acquired epileptiform aphasia are extremely variable and heterogeneous. This is in part because of the varied definitions of these conditions. Tuchman (22) defines LKS as an acquired aphasia in association with abnormal EEG readings, including spikes, sharp waves, and spike-wave discharges, which are usually bilateral and occur predominantly over the temporal and parietal regions. According to Tuchman, the term acquired epileptiform aphasia is now equated with LKS. No single EEG pattern is said to include all cases of acquired epileptiform aphasia reported in the literature (23). A controversy surrounds whether LKS should include all children who have language regression with an epileptiform EEG, including those with CSWS or autism (22). CSWS reported by Patry et al (24) in 1971 was considered by Morrell et al (4) as an essential criterion for LKS. Others suggest that LKS and CSWS are overlapping syndromes on the same spectrum, with aphasia being present in CSWS when the epileptiform activity involves the language areas (8, 25). The diagnosis of LKS in our patients was based on acquired aphasia in the setting of previously normal language development associated with MEG or EEG epileptiform activity in or near eloquent speech areas. Other suggested overlapping syndromes include rolandic epilepsy, autistic regression, and disintegrative regression (3, 22). In LKS, the regression typically involves language only and the spikes, although variable, are predominantly central and temporal. In autistic epileptiform regression, there is autistic behavior in addition to language loss and the spikes are predominantly centrotemporal. In disintegrative epileptiform regression, there is regression of language, behavior, and cognitive function and the epileptiform activity tends to be more global (22).
In general, the four children with non-sylvian spikes displayed various other cognitive or behavioral dysfunctions in addition to aphasia. Of these four patients, two were considered to have LKS on the basis of clinical findings, with CSWS in one. One of these two had bilateral midfrontal spikes and a pervasive developmental disorder. Normal milestones were reached until the age of 3 years, when the patient developed regression of speech and self-help activities and became isolated and aloof (case 14). The other had left centroparietal and posteroparietal spikes and presented with visuospatial problems and agnosia in addition to aphasia (case 8). One patient with a clinical diagnosis of LKS versus autism had left midfrontal spikes. This patient had difficulty with attention span, self-stimulatory play, and clear gaze avoidance. Unlike a child with typical autism, she was “warm and cuddly” in her interactions (case 2). Her twin sister had normal communication skills. One patient with questionable LKS had CSWS revealed by previous EEG and right parietal occipital spikes at MSI. This patient had attention deficit hyperactive disorder with problems of aggressive behavior, loss of personal hygiene, and left hemiparesis. Although there has been a definite deterioration in language skills, the patient is still able to communicate verbally and repeat full sentences (case 12). A possible explanation for the lack of perisylvian spike detection in these patients is sampling error, with perisylvian spikes not occurring during the time of MEG data acquisition but being present at other times during deep sleep. Others include secondary propagation to language areas not detected by MSI, regression of the epileptiform activity, and an incorrect diagnosis of LKS. No specific differences were noted between children with bilateral and those with unilateral involvement.
Lewine et al (26) presented the MEG findings of six children with classic LKS and 20 with acquired developmental aphasia. All six children with classic LKS were reported to show CSWS during sleep, involving the superior temporal gyrus in five and the angular and supramarginal gyri in one with propagation to the temporal lobe. Sixteen (80%) of the 20 patients with acquired developmental aphasia showed occasional epileptic spikes during sleep. In nine of these children, the spikes occurred in either the right or left superior temporal gyrus.
Morrell et al (4) reported the cases of 14 children with LKS treated with multiple subpial transections and illustrated MEG findings in two children, both of whom showed perisylvian, temporal spikes. In 11 of 14 patients with bilateral symmetric discharge EEG pattern, they were able to show a unilateral generator by using methohexital suppression. Paetau et al (27) reported MEG findings in four patients with LKS, all of whom were found to have the earliest spike activity originating in the intrasylvian cortex. They detected secondary spikes in the ipsilateral perisylvian, temporo-occipital, and parieto-occipital areas within 10 to 60 ms. In one patient, the activity had spread to the contralateral sylvian cortex within 20 ms. In two of the patients, the primary spike generator was determined to be unilateral, whereas in the other two, bilateral independent activity was found.
An underlying cause of the epileptiform activity in LKS has not been elucidated. MR imaging studies, surgical resections (5, 7), and biopsies (28, 29) are typically normal. Rarely, causative factors have been identified. Cortical biopsies in one report (30) did show evidence of slow virus infection. Other case reports have been associated with toxoplasmosis (31), otitis media (32), cysticercosis (33), arteritis (34), inflammatory demyelinating illness (35), and temporal lobe tumor (36, 37). In another report, interictal single-photon-emission CT showed unilateral left temperoparietal hypoperfusion and positron emission tomography showed bilateral hypermetabolism (38).
Although the seizure disorder tends to remit in LKS, the prognosis for language recovery is variable. Some children recover completely from their aphasia, whereas in others, severe aphasia persists into adulthood. Most patients show marked improvement but still have some linguistic dysfunction (6). Mantovani and Landau (39) reported the follow-up findings of nine patients, obtained 10 to 28 years after the onset of aphasia. Four patients had full language recovery, one had mild language dysfunction, and four had moderate language dysfunction (39).
Several factors have been reported to influence language prognosis negatively. These include early age of onset, high frequency and long duration of epileptiform discharge in language areas, and spread to homologous cortex (6). It has been hoped that earlier diagnosis and antiepileptic treatment might improve the long-term prognosis, although success has been variable (6). Morrell et al (4) reported return of speech in 11 (79%) of 14 patients who had been aphasic for at least 2 years and were subsequently treated with subpial intracortical resection (4). This surgical technique has only recently been applied to children with LKS and is still considered an investigational rather than a definitive form of treatment.
The role of MEG in evaluating patients with epilepsy continues to evolve. MEG is sensitive to epileptic activity originating from the depths of sulci, such as the sylvian fissure, because it reveals magnetic fields generated by transmembrane synaptic currents oriented in a plane tangential to the skull surface. The precise localizations afforded by MEG compared with EEG can sometimes obviate the need for invasive video EEG recording as well as direct the placement of intraoperative electrodes when surgery is being considered for patients with medically intractable epilepsy. Specifically, for patients with LKS, MEG is useful in confirming the diagnosis and may prove useful in preoperative localization if subpial intracortical transection becomes widely accepted for the treatment of intractable cases.
Footnotes
↵1 Address reprint requests to David F. Sobel, MD, Division of Neuroradiology, Scripps Clinic, 10666 N. Torrey Pines Road, La Jolla, CA 92037.
References
- Received April 21, 1999.
- Copyright © American Society of Neuroradiology