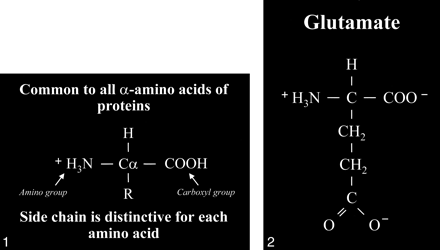
fig 1.
Amino acid. Typical amino acid consists of a central alpha carbon (Cα) that is bonded to a carboxyl group (COOH) on one side and an amino group (H3N) on the other side. Each amino acid is characterized by a distinctive molecular group (R) or side chain attached to the α carbon.
fig 2. Glutamate. The side chain, or R group, of glutamic acid is CH2CH2COOH. The carboxyl group of the side chain is designated the γ carboxyl group, which becomes fully ionized at neutral pH and is therefore frequently written with a negative charge (COO−). The term glutamate (instead of glutamic acid) is used to indicate this negative charge or ionized state at physiological pH.