Abstract
Summary: We describe a giant left orbital vascular malformation that was treated with both percutaneous and transarterial embolization. Feeder artery aneurysms thrombosed as a result of retrograde reflux of embolic material into the distal ophthalmic artery. In this presentation, we emphasize the efficacy of percutaneous embolization and retrograde thromboses of two intradural saccular ophthalmic artery aneurysms.
In the head and neck region, the discussion of the vascular malformations includes arteriovenous fistulae, as well as arteriovenous, capillary-venous, and pure venous malformations (hemangiomas). Treatment of these malformations is most challenging because of the high density of the vital structures in this region and the need to preserve function and aesthetic requirements (1).
In the past, treatment of arteriovenous vascular malformations of the scalp and face was primarily reliant on surgical excision or ligation of the feeding artery. Currently, however, therapeutic strategy is based on selective embolization, surgical excision, or a combination of both, as well as various adjunctive procedures (2, 3). Also, with the advent of the endovascular treatment techniques and new embolic agents, embolization has become the treatment of choice for these lesions (4).
Traditional access methods for interventional neuroradiologic procedures involve the use of catheter and guidewire systems. In addition, direct access to many head and neck lesions is possible through percutaneous and intra-oral routes (1).
Case Report
A 55-year-old man was referred to our department for preoperative embolization of a left orbital disfiguring mass (Fig 1). A physical examination revealed a soft, multilobular, red-purple orbital mass and that the left eye was blind. The patient reported no apparent lesion at the time of birth and denied any history of trauma.
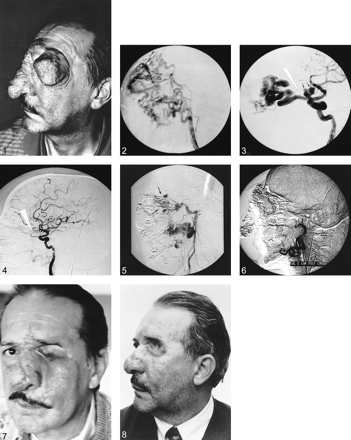
Pre-embolization photograph of left orbitonasal region shows large, disfiguring orbital vascular malformation.
fig 2. Lateral view angiogram of the left external carotid artery shows large dilated anterior branches of the external carotid artery, some blush, and early venous drainage.
fig 3. Lateral view angiogram of the left internal carotid artery injection reveals that a large dilated ophthalmic artery feeds the vascular malformation and two intradural aneurysms at its origin (arrows).
fig 4. Lateral view angiogram of the left internal carotid artery, obtained after embolization, shows that the left ophthalmic artery and two saccular aneurysms are no longer visible. During embolization, we fluoroscopically observed retrograde reflux of embolic material into the distal ophthalmic artery, so it is not visible in this figure. Some reflux of contrast material into the anterior branches of the external carotid artery is seen.
fig 5. Lateral view angiogram of the left external carotid artery, obtained after percutaneous embolization, shows residual malformation (arrows).
fig 6. After additional transarterial embolization, angiogram of the external carotid artery shows complete occlusion of the vascular malformation.
fig 7. Postoperative photograph obtained 1 week after surgery. Early postoperative edema is seen in the orbitonasal region.
fig 8. Postoperative photograph obtained 3 months after surgery. Complete resolution of the edema is seen
To evaluate the lesion before treatment planning, we performed bilateral selective angiography of the internal and external carotid arteries. The angiogram revealed a large arteriovenous vascular malformation in the left orbital region, fed by the left ophthalmic artery and anterior branches of the external carotid artery. The main feeder was the ophthalmic artery, and it had two saccular intradural aneurysms at its origin (Figs 2 and 3). We documented the location, size, feeding arteries, and venous drainage of the vascular malformation. After complete evaluation, we decided to perform direct percutaneous embolization because there were multiple fistula connections.
For this purpose, the lesion was punctured with a 20-gauge needle in the area nearest the arteriovenous connection. Angiography of the hand was then performed, with compression of venous drainage in the face and neck, to document the components of the arteriovenous malformation, to rule out supply to normal scalp, to rule out dangerous anastomoses, and to define venous outflow. After complete angiographic evaluation of the arteriovenous vascular malformation, embolization was performed using a mixture of n-butyl cyanoacrylate (Histoacryl-Blue; Braun, Melsungen, Germany) and iodized oil (Lipiodol; Laboratoire Guerbet, Roissy, France) (2 cc of Histoacryl and 8 cc of Lipiodol; 20%). The concentration of the n-butyl cyanoacrylate-iodized oil mixture was adjusted according to the flow rate, as evaluated at angiography, and the injection rate and volume were controlled by using fluoroscopic road mapping during n-butyl cyanoacrylate injections.
During the first session, we intentionally tried to mix reflux into the distal ophthalmic artery because the left orbit was blind. To do this, we injected embolic material very slowly and under very careful fluoroscopic guidance to prevent embolic material reflux into the internal carotid artery. Once we saw the reflux of mixture into the distal ophthalmic artery, we waited until it polymerized. After the first session, a control angiogram of the left internal carotid artery showed that the ophthalmic artery no longer fed the malformation and that the two saccular intradural aneurysms had disappeared (Fig 4). We think that the feeder artery aneurysms thrombosed as result of reduction of flow in the ophthalmic artery. After occlusion of the distal ophthalmic artery, six more percutaneous embolization procedures were performed to occlude other components of the arteriovenous vascular malformation. Just after seven percutaneous embolization procedures, thrombosis occurred in the lesion, which became hard. A control angiogram showed obliteration of the arteriovenous malformation at a 95% rate, with minimal residual blush due to contribution from the external carotid artery feeders (Fig 4).
After multiple direct percutaneous embolization procedures, residual components of the arteriovenous vascular malformation, which manifested itself as minimal blush on the control angiogram, were devascularized by means of additional transarterial embolization using polyvinyl alcohol particles (150−250∼t) (Fig 5). The final angiogram of the external carotid artery injection showed complete obliteration of the orbital arteriovenous vascular malformation, without any residual blush (Fig 6).
Two days later, the eyeball was exenterated, with minimal blood loss and very short operating time. The patient refused any reconstructive procedure and was discharged 5 days later. During the long-term follow-up period, we did not encounter any problems. A postsurgical photograph obtained 1 week after surgery (Fig 7) showed postoperative edema in the orbitonasal region. Three months after surgery, resolution of the edema had been achieved (Fig 8).
Discussion
Vascular malformations are composed of dysplastic vessels with normal endothelial turnover. Depending on the type of the affected vascular compartment, the flow characteristics, and the clinical symptoms, superficial vascular malformations can be classified into slow flow (hemangioma in involuting and involuted phases, capillary malformation, venous malformation, lymphatic malformation, mixed lesion with low flow components such as capillary-lymphatic malformation, capillary-lymphatic-venous malformation) and high flow (hemangioma in proliferative phases, arterial malformations, arteriovenous malformations, arteriovenous fistulae) malformations (5). Although most arteriovenous malformations occur intracranially, these lesions can occur anywhere in the body. Violaceous color of overlying skin, palpable thrill, and bruit suggest such a lesion. Ischemic and venous ulceration of skin can result from changes in venous pressure seen proximal and distal to arteriovenous vascular malformations. Puberty or other hormonal stimuli, trauma, or surgical intervention may render such lesions more evident to the patient or the physician. A large lesion can cause cardiac failure and should be investigated in appropriate clinical situations (3, 6–8).
For arteriovenous vascular malformations in the scalp, various types of treatments have been used, including surgical excision, vessel ligation, embolization, radiation, electrocoagulation, sclerosis, and compression, with the goal being complete obliteration (3). Limited surgical excision or proximal ligation may result in recruitment of collateral vessels and either enlargement of the arteriovenous vascular malformations or worsening of local ischemic changes of the overlying skin. In addition, complications of surgical management of arteriovenous vascular malformations may be as high as 28%, and persistent bleeding episodes and wound breakdown may occur (8).
Large malformations require combined repeated embolization and surgery. This combination helps the surgeon achieve adequate resection with limited blood loss. The primary goals of preoperative embolization are to diminish intraoperative blood loss and to facilitate complete surgical extirpation. Embolization should not be considered a means of reducing the extent of resection (4, 8).
The route of embolization (transarterial versus percutaneous) is chosen based on the angiographic architecture of the malformation. If there are pedicles accessible to superselective catheterization that allow sparing of the normal surrounding scalp supply and good penetration of the nidus, a transarterial approach is preferred. If the arterial pedicles are not readily accessible via a transarterial approach, the nidus and veins are accessed and embolized via a direct percutaneous route (4, 9). In all cases, selective angiography is performed before embolization to map the lesion. While performing angiography in these lesions, venous outflow can be blocked using manual compression to better delineate the internal structure of vascular lesions (4).
Preoperative transarterial embolization without superselective catheterization may be ineffective or technically difficult and can result in occlusion of the arteries that are proximal to the arteriovenous connection and of the collateral vessels that are supplied via a rich arterial network that is frequently recruited. To prevent this, the arteriovenous connection must be occluded by means of deep penetration with small particles or a liquid embolic agent, such as n-butyl cyanoacrylate mixed with iodized oil (4, 8, 9). In our case, we used both of these techniques to completely occlude the vascular malformation and we achieved an excellent result.
Direct percutaneous embolization with n-butyl cyanoacrylate is effective and safe. This technique for preoperative devascularization of arteriovenous vascular malformations in the scalp and face and in cases of superficial lesions may replace the transarterial technique because effective devascularization is immediate. With this technique, the targeted vessel is the venous structure that is just distal to the arteriovenous connection. Injection of n-butyl cyanoacrylate directly into the nidus with temporary occlusion of venous channels may have the potential risk of retrograde reflux of embolic material into the feeding artery (4). In our case, we tried to cause the embolic agent to backflow into the arterial side under very careful fluoroscopic guidance and very slow controlled injection. When we saw the n-butyl cyanoacrylate in the distal ophthalmic artery, we stopped until it polymerized in the artery to prevent any embolic agent from escaping into the intracranial circulation. As a result of the decrease of flow and reduction of caliber of the ophthalmic artery, two intradural saccular ophthalmic artery aneurysms thrombosed.
Venous occlusion during endovascular treatment of brain arteriovenous vascular malformations may cause serious hemorrhagic complications, but in cases of facial arteriovenous vascular malformations, there is no risk of such complication. During injection of n-butyl cyanoacrylate, venous outflow can be blocked using manual compression, allowing better penetration of the nidus, better filling of fistulous components, and less risk of escape of embolic material to the venous circulation. In our case, during injection of embolic material, draining venous channels located at the site of direct puncture were compressed, as revealed by angiography. Digital substraction fluoroscopy was used before injection of n-butyl cyanoacrylate and iodized oil to verify that venous outflow was completely occluded by means of manual compression. Adjunctive transarterial embolization can be performed before or after direct percutaneous embolization to slow down blood flow and allow more accurate deposition of n-butyl cyanoacrylate into the lesion (3, 4, 9).
After embolization, surgical extirpation should not be delayed any longer than 72 hr, because the resulting inflammatory reaction in the embolized area may make difficult the surgical approach. In our case, extirpation was performed with very little blood loss and a very short operating time within 24 hr (3, 10).
Actually, we think that the most interesting part of our case is association aneurysm formation between intracranial feeder artery and extracranial vascular malformation. The association of aneurysm formation with brain arteriovenous vascular malformations has been well known for some time; however, the treatment of these lesions remains controversial. According to Yasargil (11) the association was first noted by Lowes in 1925 and further documented by Stewart and Ashby in 1930 and 1931. Since that time, there have been numerous case reports and reported small series of this entity. Estimates of the prevalence of brain arteriovenous vascular malformation-associated aneurysms vary widely, with the literature quoting between 2.7% and 14% (12). To our knowledge, however, there has been no report indicating an association between extracranial vascular malformation and feeder artery aneurysm. We think this is a very rare clinical entity. The management of such aneurysms is of great debate, with some practitioners recommending embolization of the aneurysms before arteriovenous vascular malformation therapy and others recommending simply performing the arteriovenous vascular malformation therapy and allowing the aneurysms to involute on their own (11). In our case, we performed direct percutaneous embolization, intentionally trying to reflux embolic material into the distal ophthalmic artery so that the tiny embolic material could be observed on high quality fluoroscopic images. The material is not seen on Figure 6.
We achieved this backflow with very slow injection using high quality fluoroscopic road mapping. As soon as we saw the embolic material in the distal ophthalmic artery, we stopped injection and waited until it polymerized, thereby attempting to prevent embolic material from escaping into the systemic circulation. Afterward, flow in the ophthalmic artery ceased, reduction of the caliber of the ophthalmic artery occurred, and two intradural saccular aneurysms thrombosed. We then safely embolized other compartments of the arteriovenous vascular malformation.
Conclusion
We think that our case presents a rare entity that indicates an association of aneurysm formation between a feeder artery and an extracranial facial vascular malformation. Direct puncture embolization with n-butyl cyanoacrylate is an effective and safe technique for preoperative devascularization of arteriovenous vascular malformations in the scalp or face.
Footnotes
↵1 Address reprint requests to Barbaros Erhan Çil, MD, 57 sokak, 6/1 Emek, 06510, Ankara, Turkey.
References
- Received February 22, 2000.
- Copyright © American Society of Neuroradiology