Abstract
Summary: We performed MR imaging deformation-based hippocampal shape analysis in a 28-year-old woman in whom status epilepticus developed after acute encephalitis. Hippocampal shape analysis revealed severe global bilateral hippocampal atrophy. Regional volume loss was most accentuated in the medial and lateral aspects of the hippocampal head; the loss was similar to shape changes in hippocampal sclerosis of chronic temporal lobe epilepsy. This deformation pattern may reflect a common pathologic process that causes hippocampal volume loss in both of these conditions.
The emerging field of computational anatomy provides a technique for understanding the variability of brain anatomy (1). This technique provides a framework for the objective analysis of the three-dimensional shape of neuroanatomic structures. By using this framework, an electronic atlas of the hippocampus can be created as a deformable template that is then matched to an individual MR image to extract and study individual hippocampal surface structure (2). Previous reports applied this technique in patients with chronic temporal lobe epilepsy (3), schizophrenia (4), and Alzheimer disease (5), and specific areas of hippocampal shape changes were shown to affect subregions of the hippocampal head, body, and tail. Only a few reports in adults have demonstrated the development of hippocampal sclerosis after a notable cerebral insult such as status epilepticus (6, 7). In the current study, we performed deformation-based hippocampal segmentation and shape analysis to demonstrate the hippocampal surface structure in a patient with hippocampal atrophy after the development of status epilepticus associated with encephalitis.
Case Report
A 28-year-old woman who had no significant medical history presented to an outside hospital with headache, paranoia, confusion and agitation for 1 week prior to admission. The patient had no family history of seizure or febrile convulsion. CT scanning of the brain was unremarkable. Lumbar puncture showed an opening pressure of 200 mmH2O. The CSF was clear with an RBC count of 67 per cubic millimeter, WBC count of 493 per cubic millimeter (98% monocytes, 2% eosinophils), protein level of 191 mg/dL, glucose level of 65 mg/dL, and negative polymerase chain reaction findings for herpes simplex virus types 1 and 2. The patient then had a generalized tonic-clonic seizure, which was treated with intravenously administered phenytoin; the patient required intubation. Electroencephalography (EEG) showed moderate diffuse slowing. Seven days after admission, the patient remained in a coma and continued to have intermittent generalized tonic-clonic seizures despite treatment with intravenously administered benzodiazepine and phenobarbital. Repeat EEG showed prominent diffuse slowing with occasional episodes of sharp activity.
The patient was then transferred to our institution. She remained in a coma and had intermittent episodes of facial and mouth twitching. Continuous EEG monitoring at 24 hours showed episodic synchronous delta-range slowing consistent with status epilepticus. Intravenous infusions of valproic acid and midazolam were then added. T2-weighted MR imaging of the brain showed hyperintense signal involving the bilateral hippocampi (Fig 1). Forty-eight hours later, EEG monitoring showed intermittent asynchronous delta slowing. Three weeks after initial presentation, the patient remained in a coma without evidence of epileptic seizures. Repeat lumbar puncture showed clear CSF with an RBC count of 3 per cubic millimeter, WBC count of 18 per cubic millimeter, protein level of 34 mg/dL, and glucose level of 140 mg/dL. T2-weighted MR imaging of the brain showed persistent hyperintense signal in the bilateral hippocampi. One month after the onset of illness, the patient had spontaneous eyes opening and minimal response to stimulation. She was well enough for transfer to a neurorehabilitation unit 6 weeks after admission.
Sagittal fluid-attenuated inversion recovery (10,002/165/2200/1 [TR/TEeff/TI/NEX]; matrix, 256 × 192) images obtained during an episode of status epilepticus show marked abnormal hyperintense signal in the bilateral hippocampi, without involvement of surrounding structures.
A, Right hippocampus.
B, Left hippocampus.
Six months after presentation, the patient was ambulatory and living independently, but she had severe short-term memory deficits. The patient was taking valproic acid and had been seizure-free since her initial presentation. MR imaging of the brain showed severe bilateral hippocampal atrophy. Her last follow-up was 12 months after the onset of illness; during this time she had remained seizure-free.
We used MR images obtained at 9 months after onset of the illness to demonstrate the hippocampal surface structure (Fig 2). MR imaging was performed on a 1.5-T Signa unit (GE Medical Systems, Milwaukee, WI). Whole-brain acquisitions were performed in the coronal plane with the fast spoiled gradient-recalled echo (GRASS; GE Medical Systems) technique (FSPGR) with a TR/TE of 8.8/1.8 and flip angle of 30°. Voxel dimensions were 0.742 × 0.742 × 1.5 mm, the field of view was 38 × 38 cm, and the matrix size was 512 × 512. MR images from a single healthy control subject, matched for brain volume, age, and sex, was used for the comparison of hippocampal shape.
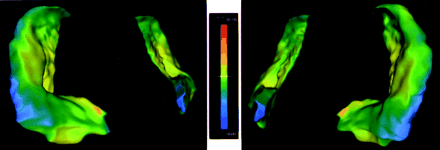
Three-dimensional deformation-based surface-rendered images of the hippocampi obtained 9 months after the onset of the acute illness demonstrate severe global hippocampal atrophy. Accentuated volume loss of the medial projection of the hippocampal head (arrow), the uncinate gyrus, is shown.
A, View from the right side. The near image is the right hippocampus, and the far image is the left hippocampus.
B, View from the left side. The near image is the left hippocampus, and the far image is the right hippocampus.
Both case and control images were processed with deformation-based segmentation of the hippocampus by using a previously described technique (2). The hippocampal segmentations of the patient were then coregistered with those of the control subject (3). Differences in the coregistration between the case and control images (deformation amplitude) were then projected on the control hippocampi. A color scale represents the deformation amplitudes, as depicted in Figure 3.
MR imaging deformation-based hippocampal shape analysis demonstrates the maximum accentuated inward deformation in the medial and lateral aspects of the hippocampal head, which are purple and blue. The magnitudes (in millimeters) and the direction of surface displacement are indicated by using a color-flame scale.
Left, View from the right side. The near image is the right hippocampus, and the far image is the left hippocampus.
Right, View from the left side. The near image is the left hippocampus, and the far image is the right hippocampus.
The hippocampal volumes of the patient were 925 mm3 on the right and 959 mm3 on the left, whereas the control hippocampal volumes were 2208 mm3 on the right and 2417 mm3 on the left. Brain parenchymal volume was 106 cm3 for the patient and 102 cm3 for the control subject.
The right hippocampal abnormalities showed maximum accentuated inward deformation in the medial and lateral aspects of the hippocampal head. The deformation maximum amplitude was 3.26 mm, the minimum amplitude was −7.73 mm, and the surface area was 1578 mm2. The surface areas with deformation amplitudes less than 1.0, 2.0, and 3.0 mm were 762, 215, and 67 mm2, respectively. The average displacement was −0.98 mm.
The left hippocampal abnormalities were most accentuated in the medial and lateral aspects of the hippocampal head. The deformation maximum amplitude was 4.21 mm, the minimum amplitude was −9.75 mm, and the surface area was 1,645 mm2. The surface areas with deformation amplitudes less than 1.0, 2.0, and 3.0 mm were 789, 360, and 160 mm2, respectively. The average displacement was −1.15 mm.
Discussion
In our patient, the initial T2-weighted MR images showed signal intensity changes that involved the bilateral hippocampi. These changes likely represented edema, which appeared during an episode of status epilepticus associated with encephalitis. Subsequently, severe bilateral hippocampal atrophy developed, as shown on MR images obtained 9 months after the onset of illness. Whether the hippocampal edema and subsequent atrophy were due to encephalitis, as suggested by the CSF profile, or whether it was secondary to prolonged seizures remains undetermined. However, unilateral or bilateral hippocampal damage in viral encephalitis, such as herpes encephalitis, rarely occurs in isolation, and it is often accompanied by lesions in other temporal regions (8). Therefore, the encephalitis alone is less likely to be responsible for the radiologic changes seen in our patient.
Pathologic studies in patients with status epilepticus without systemic complications or preexisting epilepsy demonstrate widespread neuronal damage in the hippocampus, amygdala, thalamus, and cerebellum (9). Such damage is most likely secondary to excitotoxic mechanisms as shown in domoic acid–induced status epilepticus (10). MR images obtained in our patient during an episode of status epilepticus, however, showed isolated hippocampal changes, without any abnormalities in surrounding structures. This finding is consistent with that of previously reported imaging studies of status epilepticus (6, 7, 11, 12), which mainly describe reversibly increased signal intensity in the medial temporal lobe on T2-weighted MR images. Because of the high density of glutamate receptors in the hippocampus, especially in hippocampal CA1 region, this structure tends to be affected in status epilepticus, with extensive destruction of hippocampal neurons (13, 14). The variability of structural abnormalities described in status epilepticus probably depends on the timing, severity, and cause of status epilepticus.
The application of deformation hippocampal shape analysis results in better localization of hippocampal neuroanatomic abnormalities and enables the evaluation of local details of hippocampal anatomy that may not be evident in measurements of total hippocampal volume (2). Application of this technique to specific disease states, such as schizophrenia (4) and Alzheimer disease (5), produce deformation patterns distinctly different from those of control subjects. Csernansky et al demonstrated hippocampal volume loss in the superior and lateral aspects of the hippocampal head in patients with schizophrenia (4) and a loss of tissue in the head and on the lateral surface of hippocampal body in patients with Alzheimer disease (5). Hogan et al (3) performed the same technique in patients with unilateral hippocampal sclerosis and demonstrated accentuated volume loss in the medial and lateral aspects of the hippocampal head. Their hippocampal shape analysis was different from that in a previously described in case of schizophrenia (4) and Alzheimer disease (5). This observation suggests that different disease processes may affect different areas of the hippocampus and that abnormalities in different areas of the hippocampus may contribute to the different clinical manifestations.
In the current case, we demonstrated and quantified changes in shape of the hippocampus after an episode of status epilepticus associated with encephalitis by comparing the hippocampi of our patient with those of a single matched control subject. However, the examination of a group of control subjects, rather than a single control, matched for age, sex, and brain parenchymal volume, would help in eliminating possible individual variations in hippocampal shape between the patient and control subjects. Several methods of normalization of hippocampal volumes are possible by comparing these volumes to total brain volumes (15). With any of these methods of normalization, our patient had striking hippocampal volume loss. Because of the profound hippocampal volume loss in our patient, we do not believe that the shape changes that we describe are related to individual variations of hippocampal shape between the patient and the control subject. However, we present our results only as initial findings that need to be verified with results in a larger group of patients.
Our result shows that the most accentuated regional volume loss occurred in the medial and lateral aspects of the hippocampal head (Fig 3). As previously mentioned, the CA1 region tends to show the most substantial histologic change in status epilepticus–induced hippocampal lesions (13). CA1 underlies the lateral hippocampal surface over the body of the hippocampus (14). This relationship is maintained over the lateral hippocampus at the junction of the body and head and may explain the accentuated volume loss in the lateral head region. The uncinate gyrus is the most medial region of the hippocampal head, and it is contiguous with the ambient gyrus of the amygdala. This gyrus is composed of components of CA1 and the subiculum (14), which may account for its accentuated involvement. Although both hippocampi are markedly and globally atrophic, the most prominent areas of volume loss in the hippocampal head were in similar regions, as previously described in patients with chronic temporal lobe epilepsy and pathologically confirmed hippocampal sclerosis. This deformation pattern may reflect a common pathologic process that causes hippocampal volume loss in both status epilepticus associated with encephalitis and in chronic temporal lobe epilepsy. Further studies involving the use of deformation-based segmentation for comparisons among patients with epilepsy of different causes may help to delineate the pathogenesis of hippocampal changes in epilepsy.
References
- Received August 23, 2001.
- Accepted after revision February 11, 2002.
- Copyright © American Society of Neuroradiology