Abstract
Summary: We present a case in which a stent-graft was used to treat an aneurysm of the vertebrobasilar junction. According to our literature search, this is one of the first cases involving the intracranial placement of a stent-graft and the first case in which an aneurysm of the vertebrobasilar junction was treated in this manner. A stent-graft can be useful device for the neuroendovascular treatment of aneurysms in select patients.
Covered stents has not yet found widespread use in interventional neuroradiology. Our successful treatment of an intracranial aneurysm by means of a stent-graft shows the potential of using this device.
Technique
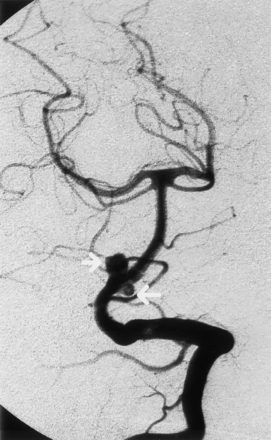
A 35-year-old man experienced a sudden severe headache. CT demonstrated subarachnoid hemorrhage of Hunt and Hess grade I–II, without motor or sensory deficits. Quantitative digital subtractive angiography (DSA) showed a wide-necked, irregularly shaped aneurysm at the vertebrobasilar junction (Fig 1). Its dimensions were as follows: dome size, 6 mm; neck width, 4 mm; left vertebral artery (VA) diameter, 4 mm; basilar artery (BA) origin diameter, 3.8 mm; right VA diameter, 1.6 mm; and body-to-neck ratio, 1.5 mm.
Initial DSA with left VA injection shows the wide-necked, irregularly shaped aneurysm of the vertebrobasilar junction (arrows)
The aneurysm was in an area that was difficult to access. Therefore, endovascular treatment was considered the therapy of choice. An analysis of the geometry of the aneurysm (irregular form, wide neck) suggested a high risk with coil embolization (1, 2). Balloon test occlusion (BTO) of the left VA was performed distal to the posterior inferior cerebellar artery (PICA). BTO lasted 25 minutes and was well tolerated. Taking into consideration the results of BTO and the favorable anatomic conditions for stent-graft placement, we considered the endovascular approach the method of choice.
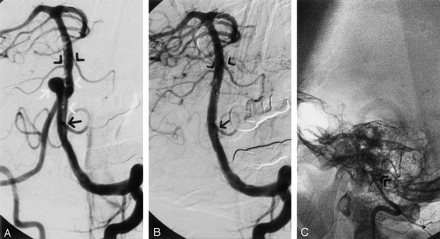
According to the protocol for stent placement, 250 mg of tyclodipin twice a day and 325 mg of aspirin once a day were prescribed 72 hours before the intervention. After stent placement, the patient received 250 mg of tyclodipin twice a day and 325 mg of aspirin once a day for 3 months, in addition to low-weight heparin of a standard dose (0.3 mL Fraxiparine twice a day) for 5 days. The procedure was performed under local anesthesia and intravenous sedation. A 8F introducer was positioned in right femoral artery by using a Seldinger technique. The patient received a bolus of heparin (5000 IU intravenously), and intermittent doses of heparin were given throughout the procedure. A 7F, 90-cm guiding catheter (Vista Brite Tip Multipurpose; Cordis Europa N.V., Roden, the Netherlands) was positioned in the distal left VA. An 0.014-in microguidewire (ATW; Cordis) was passed into the left posterior cerebral artery as distally as possible to provide support. A 12 × 4-mm stent-graft (Jostent Coronary Stent Graft Supreme System with JET coating; Jomed. Unterschleibheim, Germany) was passed over the microguidewire and carefully positioned at the level of the aneurysm, distal to the origin of the left PICA and proximal to the origins of both anterior inferior cerebellar arteries. The stent-graft was then deployed across the neck of the aneurysm and the vertebrobasilar junction (Fig 2A). This system consisted of a premounted stent made of ultrathin (75 μm) polytetrafluoroethylene (PTFE) graft material layered between two stents with reduced wall thickness. The balloon was inflated to 11 atm. DSA with left VA injection showed total closure of the aneurysm and good patency of the VA, BA, and intracranial vessels (Fig 2B). The balloon and microguidewire were removed. DSA with right VA injection showed termination of the right VA with the PICA (Fig 2C).
Coronary stent-graft placement to treat an aneurysm of the vertebrobasilar junction.
A, Contrast material injection from the guiding catheter. The stent-graft (white arrowheads) is situated at the level of the aneurysm (white arrow), distal to the origin of the left PICA (black arrow) and proximal to the origins of both anterior inferior cerebellar arteries (black arrowheads).
B, DSA with left VA injection from the guiding catheter shows total closure of the aneurysm and good patency of the VA, BA, left PICA (arrow), and origins of both anterior inferior cerebellar arteries (arrowheads).
C, DSA with right VA injection shows termination of right VA with the PICA (arrowheads).
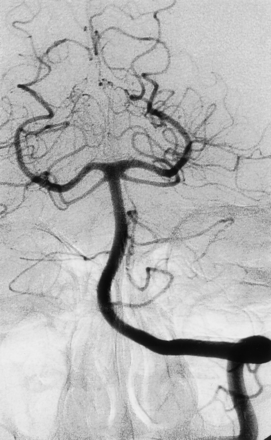
No technical complications or neurologic sequelae were observed. Follow-up angiography performed 6 months after stent placement showed no filling of the aneurysm and normal patency of the VA and BA, with no evidence of intimal hyperplasia or concomitant vessel stenosis (Fig 3).
Follow-up angiogram obtained 6 months after stent placement shows an absence of filling of the aneurysm, normal patency of the VA and BA, and no evidence of intimal hyperplasia or concomitant vessel stenosis.
Discussion
Stents have been used in the treatment of aneurysms in the last decade. There are several modalities of stent use in such treatment. Use of a stent alone to reduce blood flow in the sack and to create a matrix for endothelial growth is not efficient because of the high porosity and the need for systemic anticoagulation to reduce the risk of thromboembolic complications and subacute stent thrombosis (3, 4). Stent placement can be successful in conjunction with coil embolization (stent-supported coil embolization) (4, 5). In this situation, the stent acts as a rigid endoluminal scaffold to prevent coil herniation into the parent vessel and possibly provide an endoluminal matrix for endothelial growth. This method has shown good results, but some limitations may be encountered. For example, a microcatheter is often navigated through the stent interstices only with great difficulty (5). Also, complete obliteration of an irregularly shaped, wide-neck aneurysms is difficult, even with the combined stent placement-coiling approach. Finally, stents are known to induce intimal hyperplasia, which can result in hemodynamically significant stenosis (6).
In recent years, the use of covered stents has became a promising alternative to other endovascular techniques in select clinical situations. Covered stents have been used to occlude parent vessels (7) and to treat carotid-cavernous fistulas (8), carotid blowout syndrome (9), and fusiform dissecting aneurysm of the VA (10) while saving vessel patency. These reports suggest that the use of stent-grafts may be the easiest, fastest, and most efficient way to preserve a parent artery.
PTFE-covered stents are effective in a variety of clinical situations, with an acceptably low incident of complications, including acute and subacute stent thrombosis (on the condition of an optimal stent opening) (11). Furthermore, the PTFE layer might reduce the rate of neointimal hyperplasia and stent-related stenosis by inhibiting the migration of inflammatory cells and by attenuating the diffusion of cytokines (8, 12).
The main technical limitation associated with the placement of stent-grafts in intracranial vessels is their limited longitudinal flexibility. Another limitation is related to the occlusion of side branches by the PTFE layer and the following cerebral ischemia.
A stent-graft can be a useful device for neuroendovascular treatment in some clinical situations (eg, small curvature of the cerebral vasculature, absence of side branches on the stented segment or the possibility to sacrifice them). Future technical developments will likely improve stent-graft design and offer more sophisticated delivery systems. The long-term follow-up results of stent-graft placement, especially in cerebral vessels, are still unknown and should be studied in clinical trials.
References
- Received September 4, 2003.
- Accepted after revision October 8, 2003.
- Copyright © American Society of Neuroradiology