Abstract
SUMMARY: In adult patients with acquired immunodeficiency syndrome (AIDS), cerebral arteritis usually takes the form of arterial wall thickening, stenosis, and occlusion, leading to cerebral ischemia and infarction. Aneurysms and intracranial hemorrhage are much less commonly associated with cerebral vasculitis. For reasons not entirely clear, this form is seen more often in pediatric patients infected with human immunodeficiency virus. We report an adult patient with cerebral aneurysmal arteriopathy who presented shortly after his AIDS-defining illness in a setting of severe immune suppression and high viral load.
Central nervous system (CNS) vasculitis in patients with human immunodeficiency virus (HIV) infection is associated with opportunistic infections, lymphoproliferative disorders, or drug abuse. Primary (idiopathic) angiitis of cerebral vasculature is rare. We report an adult patient with aneurysmal arteriopathy of major arteries around the circle of Willis, a manifestation usually found only in children with acquired immunodeficiency syndrome (AIDS). We could find only 1 other adult case in the literature that suggested a direct role of HIV in aneurysmal vasculopathy.1
Case Report
A 36-year-old homosexual man was admitted to the hospital because of cough and fever for 2 days. He had diarrhea, night sweats, and weight loss for 6 months. His HIV test results were negative 4 months earlier. He had history of autosomal-dominant polycystic kidney disease.
On admission, he was febrile with otherwise normal findings on physical examination. HIV test results were positive, with a CD4 count of 43 cells/uL (normal, 250–1200 cells/uL). HIV-1 RNA level was 298,000 copies/mL. Tests for rapid plasma reagin, cryptococcal antigen, coccidioidomycosis serology, toxoplasma immunoglobulin G (IgG) and immunoglobulin M (IgM), and legionella antigen were negative. Results of cytomegalovirus (CMV) IgG antibody test were positive, but those of IgM antibody and polymerase chain reaction (PCR) for CMV DNA were negative. Hepatitis profile showed nonreactive hepatitis A, B surface, and C antibodies, but reactive hepatitis B surface and E antigens, with normal findings on liver function tests. Results of blood cultures for bacteria and fungus were negative. Erythrocyte sedimentation rate was 95 mm/h (normal, ≤20 mm/h), and C-reactive protein was 8.2 mg/dL (normal, <0.6 mg/dL), but serology was negative for antineutrophil cytoplasmic antibodies, antinuclear antibody, and rheumatoid factor. Urine Histoplasma antigen test results were negative.
One day after discharge for diagnosis and treatment of Pneumocystis carinii pneumonia, he returned to the hospital with dysarthria, acute right-sided weakness, and right Babinski sign. Lumbar puncture revealed the following values: clear CSF with 1 white blood cell, 8 red blood cells, total protein 65 mg/dL (normal, 15–45 mg/dL), glucose 49 mg/dL (normal, 50–75 mg/dL); and negative India ink, Gram stain, and acid-fast bacilli smears. All CSF analyses were negative: routine culture, Venereal Disease Research Laboratories testing, Cryptococcus latex agglutination, Aspergillus agar gel, PCR for CMV DNA, and varicella-zoster virus.
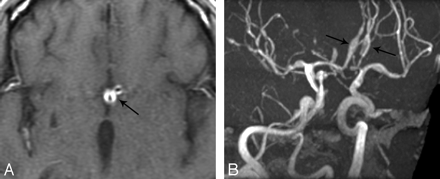
MR imaging of the brain revealed acute infarction in the posterior limb of the left internal capsule extending to the lateral thalamus and centrum semiovale. Nodular-like foci of enhancement were observed, associated with fusiform dilation of both A2 segments of the anterior cerebral arteries, with mural enhancement of both arteries (Fig 1A). MR angiography (MRA) demonstrated long-segment fusiform areas of aneurysmal dilation (Fig 1B), which alternated with areas of narrowing, most pronounced within A2 segments. Similar findings were present in the middle cerebral, posterior cerebral, and basilar arteries.
A, Axial T1-weighted image with gadolinium reveals bright enhancement of thickened walls of the anterior cerebral arteries (arrow). B, Oblique projection of an MRA demonstrates fusiform aneurysmal dilatation of the A2 segments (arrows) of both anterior cerebral arteries. Alternating areas of dilation and narrowing are also seen in the middle cerebral and basilar arteries.
Discussion
Neurologic complications in HIV-positive patients are common, and cerebral infarction is well described.2 Several factors contribute to increased frequency of cerebral infarction, but most important are infectious agents. Starting as meningitis, infectious agents directly invade leptomeningeal arteries, causing brain infarcts.
In patients with AIDS, cerebral vasculitis is a less common cause of infarction than opportunistic infections such as varicella-zoster virus, CMV, tuberculosis, cryptococcosis, and toxoplasmosis.3, 4 Pathogenesis of vasculitis results from different mechanisms, including endothelial cell infection, immune complex deposition, and impaired regulation of cytokines and adhesion molecules.4 It has been known for some time that HIV infects endothelial cells of the cerebral vasculature, leading to endothelial dysfunction.5
Our patient likely had HIV-related arteriopathy, either primary HIV vasculitis or primary angiitis of the CNS. Primary angiitis of the CNS is an uncommon disease in which the brain is the only or most affected organ. It can affect any part of the intracranial vasculature, but small arteries and veins on brain surface and leptomeninges are most commonly involved. Pathologically, granulomatous inflammatory reaction is seen, with mixed inflammatory infiltrate often accompanied by multinucleated giant cells. Primary angiitis of CNS is not specific to HIV and can be seen in other immunocompromised conditions.3, 4 Primary angiitis of CNS in HIV-infected adults has been reported.1, 4, 6 Several cases were confirmed at biopsy or autopsy,1 but most diagnoses were based on neurologic presentation, CSF, imaging, and exclusion of opportunistic infections.
In our patient, non-HIV infectious etiologies and autoimmune diseases were excluded by serologic tests, CSF analysis, and cultures. Hepatitis B virus infection can cause cerebral vasculitis,3, 7 but it is nearly always associated with polyarteritis nodosa and multiorgan involvement. Our patient had chronic Hepatitis B virus infection but was negative for hepatitis B antibody. Results of liver function tests were normal. Furthermore, clinical and laboratory data revealed no evidence of polyarteritis nodosa, and abdominal CT showed normal abdominal vessels. Intracranial aneurysms are associated with autosomal-dominant polycystic kidney disease, but typically they are saccular berry aneurysms around the circle of Willis.8
Our patient is especially interesting in that MR imaging and MRA of the brain demonstrated aneurysmal dilation of cerebral arteries, with alternate areas of narrowing, and mural enhancement with adjacent nodular enhancement. We found only 2 other adult cases in the literature with similar imaging features, but 1 patient had elevated serum antibody to varicella-zoster virus, positive varicella-zoster virus PCR, and presumed varicella-zoster virus vasculitis.6
Aneurysmal dilation of the circle of Willis and thickening of arterial walls were found in autopsy studies of children with AIDS.9–12 Pathology revealed medial fibrosis, loss of muscularis, destruction of internal elastic lamina, and intimal hyperplasia.10 One explanation for aneurysmal arteriopathy is that inflammation begins in the adventitia and involves the vasa vasorum, leading to intramural arterial ischemia. Ischemic panarteritis with injury to the vasa vasorum could lead to either aneurysmal dilation or sclerosis and stenosis.9
The affected children tended to have advanced HIV disease with severe-immune suppression.11 Cerebral aneurysms in children with AIDS have been noted to develop during periods of significant HIV replication as indicated by high serum HIV-1 viral p24 antigen levels.12
Our patient was also severely immune-suppressed with a very low CD4 count and high viral load. He had never been on antiviral therapy. An unknown factor is the quality or strain of the HIV. By history, he had multiple episodes of unprotected sex with HIV-positive men, which raises questions of multiple inoculations, strains, and higher viral load. Somewhat surprising is that our patient's aneurysmal arteriopathy was diagnosed within 1 week of his AIDS-defining infection. Although the results of an HIV test 4 months earlier were negative, he had progressive weight loss for 6 months, suggesting that he was infected earlier. Children typically have a latent period of 2–11 years after initial HIV infection, and aneurysmal arteriopathy occurs 2.5 years or longer after diagnosis of AIDS.10
In summary, although more common in children with AIDS, cerebral aneurysmal arteriopathy should be recognized as a rare but potential manifestation in adult patients with AIDS. Early diagnosis is important because treatment with highly active antiretroviral therapy and corticosteroids may stop disease progression or even induce regression of the vasculopathy.6
References
- Received August 24, 2006.
- Accepted after revision September 12, 2006.
- Copyright © American Society of Neuroradiology