Abstract
BACKGROUND AND PURPOSE: Many theories of normal pressure hydrocephalus (NPH) stress the importance of ischemia in the deep white matter. Alternate theories stress a reduction in superficial venous compliance and changes in pulse-wave propagation. An overlap in the cerebral blood flow volumes measured between NPH and controls suggests that ischemia may not be a prerequisite for this condition. This study sought to compare blood flow and compliance measures in a cohort of patients with NPH selected for having arterial inflows above the normal range to see if deep brain ischemia or superficial hemodynamic changes contribute to the pathophysiology of NPH.
MATERIALS AND METHODS: Twenty patients with NPH and arterial inflows above the normal range were selected. They underwent MR imaging with flow quantification measuring the total blood inflow, sagittal/straight sinus outflow, aqueduct stroke volume, and arteriovenous delay (AVD). Patients were compared with 12 age-matched controls.
RESULTS: The deep outflow volumes were normal. The superficial venous outflow was reduced as a percentage of the inflow by 9% (P = .04). The sagittal sinus compliance as measured by the AVD was reduced by 50% (P = .0001), and the aqueduct stroke volume was elevated by 192% (P = .02).
CONCLUSION: Ischemia in the deep venous territory is not a prerequisite for NPH. Patients with high-inflow NPH show alterations in superficial venous compliance and a reduction in the blood flow returning via the sagittal sinus. These changes together suggest that an elevation in superficial venous pressure may occur in NPH.
Normal pressure hydrocephalus (NPH) was first described 40 years ago by Adams et al1 in patients with a clinical triad of ataxia, incontinence, and dementia who also had dilated ventricles but normal CSF pressures. A rational basis for the diagnosis and treatment of this disease should rest on an adequate understanding of its underlying pathophysiology. Many physiologic changes have been noted to occur in this disease process, with ventricular dilation being the most obvious. Other changes noted are an increase in the resistance to the reabsorption of CSF,2 an alteration in the site of CSF reabsorption (ie, through the deep brain rather than through the arachnoid granulations),3,4 hyperdynamic aqueduct CSF flow,5 reduced compliance of the subarachnoid space,6,7 a normal CSF pressure but increased CSF pulse pressure (6–8 times normal),8 and an overall reduction in cerebral blood flow (CBF).9 Any theory of causation should be able to link all of these disparate findings.
Many of the current theories of NPH take the finding of a global reduction in CBF as a starting point in trying to account for the pathophysiology of this condition. Studies of many groups have shown an overall reduction in CBF in NPH.9 Most popular theories interpret the CBF reduction found in NPH to be secondary to the degree of ventricular dilation.9 The literature, however, indicates that whereas a direct correlation between reduced CBF and ventricular size has been suggested in some studies,10,11 others have refuted this finding.12,13 Mathew et al14 suggested that dilation of the ventricular system stretches the anterior cerebral arteries over the corpus callosum, thereby reducing flow. Ventricular dilation has also been suggested to increase intraparenchymal pressure and directly compress the capillary bed or venous drainage.15 Switching cause and effect, others have highlighted the finding that the vascular disease associated with ischemia actually causes the ventricular dilation by damaging the deep white matter. It is reported that watershed ischemia may exist in the deep white matter in NPH, between the boundary from the middle cerebral artery perforators and the deep medullary pial branches,14 and this leads to tissue loss. Another recent theory suggests: “With aging, the arterioles in the deep white matter close down, leading to deep white matter ischemia, which is noted with greater frequency in patients with NPH. If there is decreased arterial blood flow in, there will be less venous blood flow out, and consequently less CSF resorption via the transparenchymal/transvenous route.”15 Finally, it has also been suggested that ischemia is an epiphenomenon, occurring secondary to the stagnation of vasoactive peptides (stagnation occurs in the CSF/interstitial fluid, and the peptides are reabsorbed through the deep white matter) and that these may interfere with cerebrovascular reactivity.16
The problem with placing ischemia at the center of the causation of NPH is that not all patients have ischemia. Owler et al,17 using coregistered MR imaging and positron-emission tomography imaging data, found the CBF of the cerebrum in patients with NPH to be 24.8 ± 4.3 mL/100 g/min, with controls having a global flow of 30.5 ± 5.2 mL/100 g/min. Despite the 19% reduction in the mean CBF noted in NPH, it is apparent that the SD of the NPH data is wide enough to theoretically place 16% of patients with NPH within the normal range. This finding brings mandatory ischemia in NPH into doubt. Ischemia also fails to provide an explanation for the dynamic findings of NPH. The hydrodynamics of NPH have been shown to involve a reduction in the compliance of the subarachnoid space, brain, and the arterial tree.18 The reduced compliance directs a larger proportion of what would otherwise be a reduced total arterial pulsation toward the ventricles, increasing the aqueduct stroke volume.18 With this in mind, I compared blood flow and compliance measures in a cohort of patients with NPH, selected to have arterial inflows above the normal range, to see if deep brain ischemia or superficial venous hemodynamic changes contribute to the pathophysiology of NPH in these patients.
Materials and Methods
Subjects
A review was undertaken of all patients with a clinical diagnosis of NPH at a tertiary referral center from January 1999 to January 2005 who were evaluated with an MR imaging examination including flow quantification. One hundred thirty patients were recruited, and a chart review was performed to evaluate the presenting symptoms and lumbar puncture results. Patients with a history of significant head injury, intracranial hemorrhage, or meningitis were excluded. Planar T1-weighted sagittal and T2-weighted axial MR images were used initially to confirm ventricular dilation and exclude aqueduct occlusion. Aqueduct patency was later confirmed by the finding of hyperdynamic CSF flow on the phase-contrast aqueduct-flow studies. Of the 130 patients with a clinical diagnosis of NPH, 20 of 130 (15%) had cerebral blood inflows at or above the normal range of 700 mL/min. The high-flow NPH group consisted of 12 male and 8 female patients of a mean age 71 ± 11 years. The control group was selected from the spouses of patients and from healthy volunteers recruited via advertisement. Controls were imaged consecutively. Controls with evidence of NPH symptoms or significant structural brain lesions were excluded. In addition, each control underwent a Mini-Mental State Examination and then formal neuropsychological testing to exclude early dementia. The control group consisted of 6 women and 6 men, mean age 70 ± 5 years. Informed consent was obtained from all patients, and the hospital ethics committee approved the study protocol.
MR Imaging and Analysis
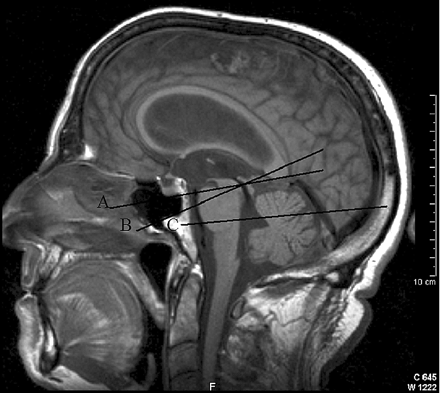
All patients were imaged on a 1.5T superconducting magnet (Magnetom Vision; Siemens, Erlangen, Germany). The patients were scanned with T1-weighted sagittal and T2-weighted axial images as well as MR flow quantification. A retrospectively cardiac-gated phase-contrast flow-quantification sequence was used with a TR of 29 ms, a TE of 7 ms, flip angle of 30°, section thickness of 6 mm, matrix of 192 × 512, FOV of 200, and a single NEX; this is a standard sequence available on this scanner. The velocity encoding (venc) values of 20 cm/s, 40 cm/s, and 75 cm/s were used. The lower venc value was selected to maximize the measurement of the aqueduct with the mid value used for the venous sinus measurements and the higher one used to maximize the arterial measurements. The 3 sections were individually positioned for each structure. The arterial plane of the section was selected to intersect the basilar artery above the superior cerebellar arteries and then pass through the vertical segments of the petrous carotid arteries (Fig 1).19 The aqueduct section was placed perpendicular to the aqueduct and midway along its length. The venous section was placed through the sagittal sinus approximately 2 cm above the torcular and then through the midportion of the straight sinus perpendicular to the flow. Regions of interest were placed around the carotid arteries, basilar artery, the sagittal sinus, straight sinus, and the aqueduct in each patient. Care was taken to exclude aliasing by retrospectively manipulating the baselines of each resultant graph, giving an effective arterial flow limit of 150 cm/s. The addition of the flow from the 3 arteries gave the total supratentorial blood inflow. The sagittal sinus and straight sinus outflow was obtained from the region of interest placed around these vessels. The percentage of the inflow represented by the outflow from both the sagittal sinus and the straight sinus was calculated. This technique has been verified both in vivo and in vitro and has been found to have error rates in the order of 5%, with a tendency to slight underestimation of flow; the intra- and interobserver variability has been described as low to negligible.20–22
A sagittal T1-weighted image of a patient with NPH, showing the flow quantification section positions: the arterial section (A), the aqueduct section (B), and the venous section (C).
The arteriovenous delay (AVD) is the time taken between the center of the arterial pulse and the center of the venous outflow pulse (measured at half height) when both are measured simultaneously, and it is inversely proportional to the pulse-wave velocity between these 2 points. The arterial pulse volume represents the degree to which the arterial tree and brain expand in systole and is calculated from the graphs obtained from each carotid and basilar artery. The mean blood flow velocity for each artery for the entire heartbeat was subtracted from the mean blood flow velocity for the period of systole for the same artery, giving the mean increase in flow velocity over systole for that vessel. This value when multiplied by the time taken for systole to occur and the cross-sectional area of the vessel (region of interest) gives the volume of expansion of that vessel in systole. This method has been previously published.19 The addition of the value of vessel expansion obtained for both carotids and the basilar arteries gave the arterial pulse volume. The aqueduct stroke volume was obtained for each patient by a process similar to that for the arterial pulse volume. By obtaining the mean flow velocity directed inferiorly and multiplying this result by the time taken for the negative flow and the cross-sectional area of the aqueduct, I obtained the stroke volume. Means and SDs were obtained for each group of patients. Differences between the groups were tested by using a nonpaired Student t test.
Results
The blood flow data are summarized in Table 1; the pulsation and compliance data are summarized in Table 2.
Blood flow
Pulsation and compliance
Clinical Findings
Twenty of 130 (15%) patients with NPH had high flow. Of the 20 patients with high flow studied, 9 (45%) presented with the entire clinical triad of ataxia, incontinence, and cognitive impairment; 5 (25%) had ataxia and cognitive impairment; 3 (15%) had ataxia and incontinence; and 3 (15%) had ataxia only. All 20 patients with high flow had a normal CSF pressure at lumbar puncture. A total of 32 of 130 (25%) of the original patients with probable NPH were offered surgery and underwent shunt insertion (this decision was usually made on the basis of a positive high-volume tap test). Of these patients, 8 of 32 (25%) who had a shunt procedure had high cerebral inflow. Following shunt surgery, 6 of 8 (75%) of the patients with high flow and 14 of 24 (58%) of the patients with low-flow NPH showed improvement in at least 1 of their symptoms.
Hemodynamics and Blood Flow
Cerebral blood inflows in the high-flow NPH group ranged from 700 to 1046 mL/min with the mean flow being 800 ± 90 mL/min (ie, 13% above the control group's value of 710 mL/min [P = .02]). This finding compares with the remaining 85% of patients who had low-flow NPH, in which the mean flow was 490 ± 100 mL/min. No significant differences were noted between the sagittal sinus or straight sinus outflows between the patients with high-flow NPH and the control group. The sagittal sinus outflow as a percentage of the inflow for the high-flow NPH group was 9% lower than that of the control group (P = .04), with the straight sinus outflow percentage being not significantly different.
Hemodynamics, Pulsation, and Compliance
The compliance of the venous system as measured by the AVD was reduced by 50% (P = .0001). The arterial expansion in systole represented by the arterial pulse was not significantly different between the groups. The aqueduct stroke volume was increased by 192% (P = .02).
Discussion
Most authors take the view that the diagnosis of idiopathic NPH is made on clinical grounds alone and that supplemental tests are not intended to diagnose NPH per se but rather to diagnose shunt-responsive NPH.15 Edwards et al3 in their review of hydrocephalus note, “Gait disturbance is the principal symptom of NPH, and it is around this feature that the clinical diagnosis should be organized. Gait is the most likely symptom to improve, with the frequency of the other symptoms being variable and their presence not being a prerequisite for the diagnosis of NPH.”3,23 In addition, the extensive review of the diagnosis of NPH by Relkin et al24 recommends classifying patients with NPH into 3 groups (ie, probable, possible, and unlikely NPH). On the basis of history, neurologic examination, and confirmation of ventriculomegaly on MR imaging by using the criteria of Relkin et al, 17 of 20 of the patients included in this study had a clinical diagnosis of probable NPH and 3 of 20 patients with ataxia alone qualified as having possible NPH. Patients with cognitive impairment and ventricular dilation alone were excluded.
Ischemia and NPH
There is a high correlation between the finding of low blood flow and NPH.9 Is this cause, effect, or epiphenomena? A review of the literature on NPH and CBF would suggest that approximately 16% of patients with NPH have blood flows above the normal range.17 In the present review, 15% of patients with a clinical diagnosis of NPH had high flow on MR imaging, which is consistent with the literature. If one were to suggest that ischemia is the cause of NPH, then one could make 3 predictions: 1) Patients with low flow would have the best chance of improvement postshunt, 2) patients with high flow would have little scope to increase their flow and, therefore, have a lower chance of improvement, and 3) the change in blood flow after surgery would correlate well with the overall improvement in symptoms. All 3 of these predictions are wrong. In this study, patients with high flow were overrepresented in the patients being offered a shunt (25% versus 15%). This is probably based on a lower estimate of the burden of atrophy on the planar imaging in those with better flow (ie, patients with higher flow probably do not have significant atrophy or Alzheimer disease). As well as being overrepresented, the patients with high flow had a better response to shunt surgery than the patients with low flow (75% versus 58%); Marmarou et al25 similarly noted a better response in patients early in their disease who had presumably less comorbidity and, therefore, probably higher flows. The literature, however, suggests a poor correlation between outcome and a change in the inflow postshunt. Although some studies have shown in-creases in CBF following CSF drainage, more recent studies have found that there is no conclusive evidence of an increase in CBF post-CSF removal; therefore, this is not helpful in predicting outcome.9
Rather than global ischemia, one may hypothesize that focal ischemia (eg, in the basal ganglia and/or deep white matter) may be the cause of NPH. Owler et al17 found that although there was no significant difference in the regional values of CBF between the white matter regions,17 a more detailed study by the same group did show a reduced CBF in the deep white matter in NPH adjacent to the ventricles, which returned to the normal range with increasing distance from the ventricles.26 Similarly, they noted a significant reduction in the CBF in the thalamus and basal ganglia in the NPH. However, they later noted, “Whether the reduction in the CBF of the basal ganglia and thalamus is a primary phenomenon or it occurs secondary to deafferentation remains to be elucidated.”17 The current study shows both the total flow and the percentage return coming from the deep system (ie, draining both the basal ganglia and deep white matter through the straight sinus) to be normal in the patients with high-flow NPH, indicating that they do not have deep ischemia. It could be argued that a measure of the total blood flow coming from both the basal ganglia and deep white matter (straight sinus flow) may mask or underestimate the degree of white matter ischemia. For this to occur, with a normal straight sinus flow, then the basal ganglia would need to be hyperemic to make up the shortfall in the flow from the deep white matter. Given the findings of Owler et al,17 basal ganglia hyperemia does not appear to be a tenable hypothesis in any patient with NPH.
It has been shown that the ischemia that does occur in patients with NPH is likely to be secondary to the reduced requirement for nutrients due to deafferentation and reduced neuronal activity rather than the primary event. In the normal human brain, regional CBF is closely coupled to regional cerebral oxygen use, so the fraction of the arterial oxygen extracted (OEF) by both white and gray matter is similar and is normally approximately 40%.27 Brooks et al27 studied the OEF in patients with NPH and found that the reduction in CBF noted in patients with NPH was directly matched to the requirement for oxygen giving a normal oxygen extraction of 40%. This implied to the authors that the reduced CBF seen in NPH was a result of neuronal loss or neuronal hypometabolism rather than the cause of the neuron loss or hypometabolism.27
Compliance and NPH
Patients with high-flow NPH do not have ischemia; however, there remains evidence of superficial venous hemodynamic change. Previous studies of NPH associated with ischemia have noted a 50% reduction in venous compliance and a 28% increase in sagittal sinus blood flow (but no change in straight sinus blood flow) following shunt surgery.6 This study of high-flow NPH similarly shows a 50% reduction in sagittal sinus compliance as measured by the AVD and alterations in the superficial but not deep venous blood flow. The compliance of a vessel can be measured noninvasively. Aortic compliance is estimated by measuring the time the pulse wave takes to travel the length of the body by using brachial and ankle artery waveforms.28 The pulse-wave velocity is inversely proportional to the compliance of the vessel it travels through. Similarly the time a pulse wave takes to pass from the arterial side to the venous side of the intracranial tree measures the compliance between these 2 sites. From Table 2, it is noted that the AVD and compliance are reduced by 50% in NPH compared with normal values (P = .0001). Because 80% of the vascular compliance is on the venous side of the vascular tree,29 the reduced compliance noted must be predominately from the venous side. Recently, Mase et al30 have confirmed a reduced compliance in NPH by using a more complicated MR imaging technique than that used here and have shown a 64% reduction in compliance in NPH, which is similar to my findings presented previously.
The reduced venous compliance changes the pulse propagation throughout the supratentorial cavity. The arterial pulse is the amount of extra blood entering the arterial tree in systole, over and above the mean (or average) flow, and this is the impetus for both ventricular and cortical vein compression, as well as displacement of CSF into the spinal canal.18 It can be seen in Table 2 that even though the impetus for ventricular compression (the arterial pulse) is normal in high-flow NPH, the aqueduct flow is increased by 192% (P = .02). This indicates that a hydrodynamic shift must occur with less compression of the cortical veins and more compression of the ventricles. Cortical vein compliance has been shown to be reduced before shunt surgery and significantly increased after shunt surgery in a cohort of patients with NPH, indicating the compliance changes noted in these veins are functional not structural.7 The cortical veins may become functionally incompliant if the venous pressure is increased because the vein will be taken to a less compliant point on its pressure volume curve. If an elevation in pressure occurs in the sagittal sinus in NPH, this will also elevate the cortical vein pressure q.v.
Venous Pressure Elevation in NPH?
The blood flow data show a paradoxic reduction in superficial drainage rather than deep drainage from the brain in NPH. The literature has noted that when ischemia does occur, it preferentially affects the deep white matter and the basal ganglia,17 not the superficial brain. Despite these findings, there is evidence in this cohort without ischemia of a 9% (800 × 0.09 = 72 mL/min) reduction in sagittal sinus blood flow in NPH compared with the normal 44% venous return. The literature suggests that when low blood flow does occur in NPH, it is probably fixed (due to atrophy or Alzheimer disease) because the blood flow does not appear to increase following shunt insertion.12,31 Despite this finding, a previous MR imaging study has shown a 28% (49 mL/min in this cohort) increase in blood returning from the sagittal sinus but no change in straight sinus following shunt insertion. 6 A review of the arterial inflow data from this original study did not show a significant change in inflow following shunt surgery (unpublished data, Bateman 2000), and this indicates that the 49 mL/min increase had to come from somewhere other than extra inflow.6 If the missing 72 mL/min from the current cohort was added to the sagittal sinus outflow once these patients underwent shunt surgery, then the sagittal sinus flow would increase by 26%, which would be almost identical to the finding of the previous studies.
If the inflow to the brain is normal, where does the 72 mL/min not returning via the sagittal sinus go? The answer is that the venous outflow can vary, despite a normal inflow, depending on the venous outflow pressure. This has been documented in idiopathic intracranial hypertension, in which elevated venous pressures have been shown to reduce the percentage venous return by promoting collateral flow.32 The flow in collaterals is not accounted for by the sinus measurements. Therefore, the data suggest that a small pressure elevation may occur in the sagittal sinus (but not in the straight sinus) in NPH, which encourages, on average, 72 mL/min to return via collateral flow. Venous pressure elevations have been documented in children with both communicating and noncommunicating hydrocephalus, and these pressure elevations are high enough to stop CSF reabsorption occurring via the arachnoid granulations.33,34 The venous pressures in children were reduced in a linear fashion once the CSF pressure was reduced by CSF aspiration.33 The increase in sagittal sinus flow noted to occur in patients with shunts and NPH (despite the lack of evidence of a change in inflow) suggests a reduction in venous pressure (similar to that occurring in children) and the abolition of the collateral flow following shunt surgery.
The reason that the elevation in venous pressure, noted in childhood hydrocephalus, was not further investigated was the belief that if the CSF pressure caused the venous pressure rise, then the venous pressure rise could not also be the cause of the CSF pressure rise. This statement may not be true if it can be shown that hydrocephalus alters the cranial system in such a way that a positive feedback loop develops. It has been shown by using cats as a model that CSF outflow resistance is proportional to the intracranial compliance (ie, a reduction in compliance reduces the rate of CSF reabsorption).35 Similarly, removal of the cranial vault induces the trephined syndrome in humans. Bone flap removal elevates compliance and is associated with reduced sagittal sinus and CSF pressures and increased CSF reabsorption.36 Cranioplasty increases both the sagittal sinus and CSF pressure and reduces compliance in this disorder.36 Finally, craniospinal compliance is reduced37 and sagittal sinus pressure is increased38 with normal aging. These findings together suggest a link between compliance and sagittal sinus pressure.
A Venous Hemodynamic Theory of NPH
Thus, we may develop a theory of NPH centered on a positive feedback loop. Aging causes craniospinal compliance to be reduced37 (in secondary NPH, significant hemorrhage or meningitis probably also reduces craniospinal compliance), and in those with a very low compliance, the venous pressure is significantly elevated. This is the first half of the feedback loop. An elevation in venous pressure makes the cortical veins stiffer,7 reducing craniospinal compliance further and providing the second half of the feedback loop. When the sagittal sinus pressure rises by 3–4 mm Hg, then the pressure gradient required to reabsorb CSF is exceeded and CSF absorption via the granulations ceases.39 The data suggest that in adults, the pressure in the deep veins does not become elevated to the same degree as the superficial system (there is no evidence of collateral flow in the deep system); therefore, the CSF would be preferentially reabsorbed via the subependyma into the deep system.40 The CSF pressure would not necessarily become elevated because this would depend on the pressure in the deep venous system and the resistance to flow across the ependyma. The reduced compliance in the cortical veins and the craniospinal canal would limit arterial expansion in the subarachnoid space; therefore, pulse waves would be propagated into the brain parenchyma and capillary bed.41 The brain would become hyperdynamic, and the aqueduct pulsation would be increased.18 Hyperdynamic pulsations directed centrally and focused on the subependymal white matter would disrupt the glial tissue and reduce the elastic recoil of this region,6,42 allowing ventricular enlargement to occur with little or no pressure gradient.8 Shunt insertion would break the feedback loop by increasing compliance, thus reducing venous pressure and increasing CSF reabsorption (increased flow through the normal CSF reabsorption pathway has been shown to occur following shunt insertion).43 The ventricles would not necessarily be reduced in size with shunt surgery44 because the original enlargement was caused by a loss of elastic recoil and not a pressure gradient. A large pressure gradient can occur in acute hydrocephalus but does not occur in chronic hydrocephalus.8,45 The loss of elastic recoil would not be expected to be repaired following shunt insertion.
Conclusion
Ischemia in the deep venous territory is not a prerequisite for NPH. Patients with high-inflow NPH show alterations in superficial venous compliance and a reduction in the blood flow returning via the sagittal sinus. These changes together suggest that an elevation in superficial venous pressure may occur in NPH.
References
- Received February 19, 2007.
- Accepted after revision May 24, 2007.
- Copyright © American Society of Neuroradiology