Abstract
SUMMARY: Lenticulostriate aneurysms are rare and usually present with intracranial hemorrhage, limiting understanding of their natural course. We describe an unusual case of an unruptured rapidly growing distal LSA aneurysm in the setting of Moyamoya disease, successfully embolized with n-BCA following functional neurologic testing with amobarbital.
Abbreviations
- n-BCA
- n-butyl cyanoacrylate
- MCA
- middle cerebral artery
- ACA
- anterior cerebral artery
- LSA
- lenticulostriate artery
- DSA
- digital subtraction angiography
Reports of LSA aneurysms are rare in the literature, numbering in the low 20s, with most cases diagnosed following intracranial hemorrhage due to rupture.1–5 The natural history of these aneurysms is, therefore, largely uncertain. Predisposing factors include hypertension, arteriovenous malformation, infection, systemic lupus erythematosus, and Moyamoya disease.4,5 The inherent difficulty in surgical treatment of these aneurysms, due to deep location, small size, and sometimes unfavorable morphology, is compounded in the setting of Moyamoya disease by vessel hemodynamic fragility.2,6–8 We discuss the utility of functional testing with amobarbital (Amytal) before endovascular embolization with n-BCA of an unruptured distal LSA aneurysm in the setting of Moyamoya disease.
Case Report
A 35-year-old woman with Moyamoya disease and a history of 2 ruptured aneurysms was referred for follow-up. Physical examination demonstrated mildly decreased strength on the left, decreased bilateral upper quadrant peripheral vision and right-sided hearing loss, and slightly unsteady gait. Reflexes and sensory examination findings were normal. Mental status examination demonstrated normal higher cortical and language function. The findings of the remainder of her physical examination and medical history were unremarkable.
CT angiography demonstrated a 3-mm right distal LSA aneurysm and a Moyamoya vascular pattern, with numerous small-vessel collaterals supplying the MCA and ACA territories bilaterally. A right frontal pericallosal artery branch aneurysm clip was present. Conventional angiography 1 month later again demonstrated a right LSA aneurysm, now measuring 4.2 × 3.9 × 3.8 mm with a 1.8-mm neck, and collateral supply to a posterior frontal MCA branch. She was referred for endovascular treatment 10 days later.
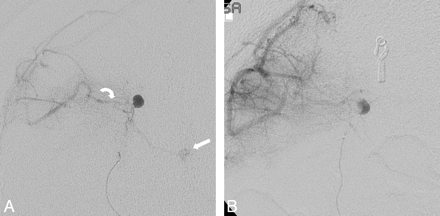
A 6F Guider catheter (Boston Scientific, Fremont, California) was positioned in the distal right internal carotid artery, through which an Excelsior SL-10 microcatheter (Target Therapeutics/Boston Scientific, Fremont, California) was advanced over a Synchro-10 microguidewire (Boston Scientific, Natick, Massachusetts) and positioned proximal to the aneurysm within the involved LSA. Superselective contrast injection demonstrated a 4.4-mm LSA aneurysm, retrograde filling of a dilated right posterior frontal MCA branch collateral from the aneurysm, and faint basal ganglia blush (Fig 1A). We administered 25 mg of Amytal via the microcatheter; subsequent neurologic examination demonstrated left facial palsy and worsening left upper extremity weakness. Intraprocedural angiography demonstrated mild vasospasm of the vessel, which improved after intra-arterial administration of a total of 150 mcg of nitroglycerin and removal of the microcatheter.
A, Pretreatment anteroposterior DSA projection of right LSA injection in the delayed arterial phase demonstrates a right distal LSA aneurysm, retrograde filling of a right posterior frontal MCA branch collateral (curved arrow) from the LSA aneurysm, and faint basal ganglia blush (straight arrow). The patient had left facial palsy and worsening left upper extremity weakness following administration of Amytal. B, Anteroposterior projection with the catheter positioned more distally in the LSA demonstrates no basal ganglia blush in late arterial phase. Amytal administration revealed no resultant neurologic deficit.
Following improvement of left facial palsy, a Spinnaker Elite 0.15 microcatheter (Boston Scientific) was advanced over a Mirage microguidewire (ev3, Irvine, California) more distally in the same vessel (Fig 1B), and an additional 25 mg of Amytal was administered. No resultant deficits were observed. The lenticulostriate vessel and aneurysm were then successfully embolized by using n-BCA, eliminating both anterograde and retrograde filling of the aneurysm (Fig 2). The patient's postoperative course was uneventful, and she was at preprocedural baseline neurologic status at discharge. At 1-month follow-up, she had no new neurologic symptoms and had begun running for exercise.
A, DSA in the delayed arterial phase after treatment demonstrates no filling of the LSA aneurysm or the right posterior frontal MCA collateral. B, Pretreatment DSA for comparison.
Discussion
A significant proportion of reported cases of LSA aneurysm are in patients with Moyamoya disease or Moyamoya-like vascular changes.1–3,9 These patients are significantly more susceptible to aneurysm formation compared with a baseline risk of 1%–6% in the general population; in a series of 81 patients with Moyamoya disease, 15% had cerebral aneurysms.7 Although most aneurysms in Moyamoya disease are in the circle of Willis (56%), a significant minority are found in the basal ganglia (18%) and collateral vessels (22%).7 Aneurysms are thought to arise in unusual branch vessels and collaterals in Moyamoya disease due to altered hemodynamics and the inherent vascular fragility. CT angiographic surveillance should, therefore, include not only the circle of Willis but the entire intracranial circulation. Although it has been suggested that most peripheral or collateral artery aneurysms in Moyamoya disease are pseudoaneurysms due to prior rupture of friable vessels,9 most reported cases of LSA aneurysms have undergone surgical treatment and have been confirmed as true aneurysms.1,2,4,5,10
Intracranial hemorrhage in Moyamoya disease, a common manifestation in adults, is associated with a high incidence of rebleeding and significant morbidity.10 In addition, the outcome of aneurysm rupture is poor in patients with Moyamoya disease, with 39% of ruptured basal ganglia aneurysms and 32% of collateral vessel aneurysms resulting in death or severe disability in 1 series of 111 patients.7 The average size of ruptured LSA aneurysms was 3.2 mm in 1 series of 6 patients and ranged from 2 to 5 mm in most reported cases in which size was reported.1,2,5,8 We observed approximately 40% growth of an unruptured LSA aneurysm during the course of 1 month, with a size within this range. Such rapid growth and historically small size at rupture underscores the need for urgent treatment of these aneurysms on diagnosis.
Most reported cases of LSA aneurysms have been treated surgically, often because anatomic considerations have rendered endovascular access difficult or impossible.1,2,4,5,10 However, improving microvascular techniques and technology, such as formable and torquable microcatheters and microguidewires, may enable more frequent endovascular treatment in the future. Although endovascular treatment may result in sacrifice of the parent LSA, a surgical approach may have the same end result, with the additional risks of damage to valuable collaterals and delayed injury to the parent LSA due to aneurysm clipping.2,7,8 In addition, the inherent difficulty in surgical treatment of these aneurysms is compounded in Moyamoya disease by vessel friability and hemodynamic fragility.2,6–8 An endovascular approach not only spares the patient a craniotomy and its attendant risks but also allows functional evaluation of the vascular territory via neurologic examination following amobarbital injection or test occlusion by catheter.6,11 This can be particularly useful in cases in which the aneurysm neck cannot be accessed by microcatheter and one is faced with sacrifice of the parent vessel, allowing avoidance of potentially devastating neurologic deficits.
In summary, distal LSA aneurysms can rapidly enlarge, commonly present with rupture, and are associated with significant morbidity and mortality. Vascular and hemodynamic fragility in Moyamoya disease complicates already difficult surgical treatment. Endovascular techniques can circumvent some inherent surgical risks and allow functional evaluation of the intended target vasculature via amobarbital or test occlusion before embolization. This is particularly useful in cases in which parent vessel occlusion might otherwise result in potentially devastating neurologic deficits.
References
- Received October 1, 2009.
- Accepted after revision November 6, 2009.
- Copyright © American Society of Neuroradiology