Abstract
BACKGROUND AND PURPOSE: Neurologic Wilson disease is an inherited disease characterized by a copper metabolic disorder that causes damage to many organs, especially the brain. Few studies report the relationships between these neurologic symptoms and MR imaging of the brain. Therefore, we investigated the correlation of brain abnormalities in patients with neurologic Wilson disease with their clinical symptoms, age of onset, and lag time to diagnosis.
MATERIALS AND METHODS: A cohort of 364 patients was recruited in China between January 2003 and December 2017. Age of onset, lag time until diagnosis, and neurologic symptoms were recorded, and cranial MR imaging was performed. Patients were divided into groups within each of these factors for correlation analysis with the MR imaging brain scans.
RESULTS: Abnormal signals in the MR imaging brain scans were seen in all 364 cases. Affected regions included the putamen, pons, midbrain, and thalamus, while the medulla and occipital lobe were unaffected. The putamen was the most frequently damaged brain region in this study. With the age of onset younger than 10 years, cranial MR imaging scans showed only impairment in the putamen. Patients with a longer lag time before diagnosis were more likely to have impairment in the pons, midbrain, and cortex. Among neurologic symptoms of Wilson disease, torsion spasm is associated with the midbrain and cortex, and choreoathetosis is related to the caudate nucleus.
CONCLUSIONS: Abnormalities in the putamen, pons, midbrain, and thalamus are part of the neuroimaging spectrum of Wilson disease. There is a significant correlation between the site of brain injury and diagnosis lag time.
ABBREVIATION:
- WD
- Wilson disease
Wilson disease (WD), also known as hepatolenticular degeneration, is an autosomal recessive disorder of human copper metabolism,1,2 caused by pathogenic variants in the copper-transporting gene ATP7B.3⇓–5 WD leads to intracellular copper accumulation, causing damage to many organs, especially the brain.6⇓–8 Neurologic WD is one of the main forms of the disease, with some patients showing severe neurologic symptoms that persist despite treatment. Further neurologic deterioration may be observed even after treatment initiation.9⇓⇓–12
MR imaging is a sensitive method to evaluate the brains of patients with neurologic WD.8,13 Whereas abnormalities in the putamen are the most common feature of neurologic WD,14 brain shrinkage is also frequently observed.15 It has been suggested that the clinical manifestations of WD are region-specific,16 and there may be specific differences among patients with WD in China. Therefore, this study aimed to explore the characteristics of cranial MR imaging among patients with WD in China. Many reports have recently published MR imaging data of patients with WD, but the sample sizes of these studies have been limited.17⇓–19 Moreover, investigations of the relationship between abnormal findings on MR imaging and neurologic symptoms in patients with WD are sparse. In this study, we conducted MR imaging in 364 patients with WD before they received any treatment for the disease. We provide a detailed report of their MR imaging in conjunction with factors such as age of onset, lag time until diagnosis, and clinical symptoms.
Materials and Methods
Study Participants
The study included 364 patients recruited from the Affiliated Hospital of the Institute of Neurology at Anhui University of Chinese Medicine between January 2003 and December 2017. Within this cohort, 219 patients were male and 145 were female. All data were collected at the time of the patient's hospitalization, and the data were summarized in a timely manner.
Diagnostic Criteria
The criteria used for recruitment to this study, as is standard for WD diagnoses, were as follows7,20⇓–22:
1 Family heredity: parental consanguinity, having a sibling with WD, or having a sibling who died from unexplained liver disease.
2 Presence of an extracorticospinal tract symptom such as dystonia, parkinsonism, chorea, torsion spasm, or liver symptoms.
3 Kayser-Fleischer rings visible to the naked eye or with slit-lamp examination.
4 Ceruloplasmin level <2.16 μmol/L or serum copper oxidase level <0.20 optical density units.
5 Urinary copper excretion level of >1.6 μmol every 24 hours.
6 Hepatic copper concentration via needle biopsy of >250 μg/g.
Participants fulfilling both criteria 1 and 4 or both criteria 2 and 4 were diagnosed with symptomatic WD. Participants fulfilling both criteria 3 and 5, only criterion 4, or only criterion 6 were diagnosed with asymptomatic WD. Only patients with symptomatic WD were included in the cohort of this study.
Study Measures and Group Analysis
Age of disease onset, diagnosis lag time (time from symptom onset to definite diagnosis), and type of clinical neurologic symptoms were recorded, and groups were established for subsequent correlation analysis. Patients were divided into 5 groups based on the age of onset, as follows: younger than 10 years, 10–14 years, 15–30 years, 31–40 years, and older than 40 years. In terms of diagnosis lag time, patients were divided into 5 groups: <3 months, 4–6 months, 7–12 months, 1–3 years, and > years. Finally, the 5 neurologic symptom groups used for correlation analysis were dystonia, parkinsonism, torsion spasm, and choreoathetosis.
MR Imaging
A 1.5T MR imaging scanner (Avanto; Siemens, Erlangen, Germany) (T1–TR = 210.0 ms, TE = 3.8 ms, time of acquisition = 46.83 seconds, bandwidth = 160.0 Hz/Px, T2–TR = 3300.0 ms, TE = 99.0 ms, time of acquisition = 56.27 seconds, bandwidth = 195.0 Hz/Px) was used to image the T1 and T2 signals in the basal ganglia, brain stem, and cerebral cortex of participants. The basal ganglia images included the putamen, caudate, and globus pallidus as well as the thalamus. Images from the brain stem included the midbrain, pons, and medulla. Scans of the cerebral cortex showed the frontal, temporal, parietal, and occipital lobes. All MR images were analyzed separately by 2 researchers (X.Y. and R.Y.). Disagreements about the findings were resolved by discussion until a consensus was reached. This MR imaging protocol was followed for the entire 15-year study period.
Statistical Analyses
The Kruskal-Wallis test was used to assess the statistical difference in the mean age of onset among the groups. The MR imaging manifestations were used to determine the statistical differences in neurologic symptoms among the groups. Continuous data with a normal distribution are presented as mean ± SD, and the frequencies of categoric variables are presented as sample size with percentage. All data were analyzed using SPSS, Version 10.01 (IBM, Armonk, New York), and P < .05 was considered statistically significant.
Results
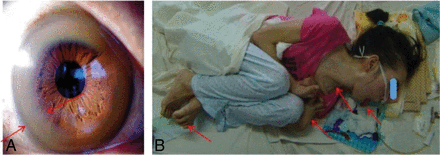
After diagnostic testing, we found that all patients showed ceruloplasmin levels of <2.16 μmol/L, serum copper oxidase levels of <0.2 optical density units, and urinary copper excretion levels of >1.6 μmol per 24 hours. All participants were positive on slit-lamp examination for Kayser-Fleischer rings (Fig 1A). In addition to the presence of neurologic symptoms, the above-mentioned values resulted in the clear diagnosis of symptomatic WD in all 364 patients included in our study. According to participants' neurologic symptoms, we found 197 cases of dystonia, 127 cases of parkinsonism, 32 cases of torsion spasm, and 8 cases of choreoathetosis (Fig 1B).
A, Kayser-Fleischer rings observable with the unaided eye (arrows). B, Wilson disease in a 23-year-old women with torsion spasm (arrows).
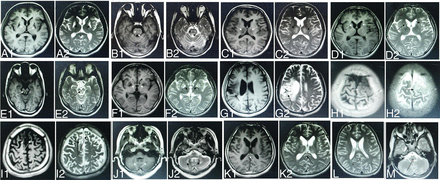
All patients underwent cranial MR imaging before their initial copper chelation therapy. The scans showed long T1 and long T2 abnormal signal in the brain (Fig 2A). All 364 patients with WD showed abnormal signal in at least 1 examined brain region (Table 1). Most patients showed abnormal signal in the following brain regions: 81.0% in the putamen (Fig 2A), 46.4% in the pons (Fig 2B), 36.0% in the thalamus (Fig 2C), 34.3% in the caudate nucleus (Fig 2D), 33.5% in the midbrain (Fig 2E), and 15.4% in the globus pallidus (Fig 2F). Brain regions that were less often affected by WD were the temporal lobe (in 3.3% of patients, Fig 2G), parietal lobe (3.3%, Fig 2H), frontal lobe (3.2%, Fig 2I), cerebellum (2.2%; Fig 2J), and corpus callosum (in 2.2%, Fig 2K). No abnormalities were observed in the occipital lobe (Fig 2L) or medulla oblongata (Fig 2M).
WD in a 14-year-old boy with dysarthria and dystonia with abnormal signal in the putamen (A1, A2). WD in a 19-year-old woman with dysphagia and dystonia with abnormal signal in the pons (B1, B2). WD in a 24-year-old woman with dysarthria and parkinsonism with abnormal signal in the thalamus (C1, C2). WD in an 18-year-old man with choreoathetosis with abnormal signal in the caudate nucleus (D1, D2). WD in a 22-year-old woman with dysarthria and parkinsonism with abnormal signal in the midbrain (E1, E2). WD in a 19-year-old woman with dystonia with abnormal signal in the globus pallidus (F1, F2). WD in a 27-year-old man with torsion spasm with abnormal signal in the temporal lobe (G1, G2). WD in a 19-year-old man with epilepsy and dysarthria with abnormal signal in the parietal lobe (H1, H2). WD in a 17-year-old boy with psychiatric symptoms with abnormal signal in the frontal lobe (I1, I2). WD in an 18-year-old man with dysarthria and parkinsonism with abnormal signal in the cerebellum (J1, J2). WD in a 13-year-old girl with parkinsonism with abnormal signal in the corpus callosum (K1, K2). WD in a 23-year-old woman with dystonia with normal signal in the medulla oblongata (L). WD in a 27-year-old man with dysarthria and dystonia with normal signal in the occipital lobe (arrows) (M).
Correlations between region-specific brain damage in neurologic WD and accompanying neurologic symptomsa
The most common areas with brain damage in our patients with WD with dystonia were the putamen, pons, and thalamus. However, cranial MR imaging of patients with WD with torsion spasm showed extensive impairment in the putamen, pons, and midbrain. Compared with patients with WD who had other neurologic symptoms, the cerebral cortex was more often impaired in the group with torsion spasm; the probability of damage in ≥1 of the cortical lobes in this group was approximately 18%–21%. Taken together, our MR imaging data show that the putamen is the most frequently damaged brain region in patients with WD, regardless of the accompanying neurologic symptoms, followed by the pons and the thalamus. Whereas the symptom of torsion spasm in WD seems to indicate abnormalities in the midbrain and cortex, the symptom of choreoathetosis predominantly suggests damage in the caudate nucleus.
In our patient cohort, age of disease onset varied from 5 to 42 years, with an average onset at 17.04 ± 6.13 years of age. Table 2 shows that the age of onset in most patients with WD was younger than 30 years (95.9% of all patients), among whom 6.9% were younger than 10 years at disease onset, 30.8% were 10–14 years of age, and 58.2% were 15–30 years of age. In the group with age of onset younger than 10 years, cranial MR imaging scans showed impairment only in the putamen. However, in the group with age of onset of 10–14 years, cranial MR imaging scans very frequently revealed impairment in the brain stem.
Correlations between age of onset and region-specific brain damage in neurologic WDa
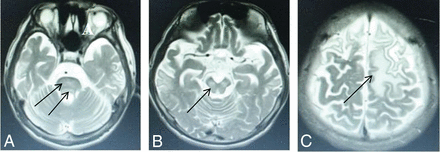
The diagnosis lag time, defined as the time between initial symptom onset and confirmed diagnosis, averaged 3.78 ± 4.46 years and varied from 1 month to 23 years. Table 3 shows that only 74 patients were diagnosed within 6 months of symptom onset. Our data showed that an increased frequency of the presence of brain damage correlated with an increased delay in treatment initiation. In the group with a lag time of <3 months, 90.6% of participants showed putamen impairment (Fig 2A) and a few had impaired thalami (Fig 2C) and pontes (Fig 2B). The latter patients had no defects in the midbrain or cerebral cortex. Our research revealed that diagnosis lag time does not correlate with impairment of the putamen in WD but does correlate with the severity of accompanying neurologic symptoms and the likelihood of impairment in the pons (Fig 3A), midbrain (Fig 3B), and cerebral cortex (Fig 3C). The P value obtained by the Fisher exact probability method was .001, and the difference was statistically significant. The MR imaging influence in the brain region was related to the course of disease.
Correlations between diagnosis lag time and region-specific brain damage in neurologic WDa
High signal intensity lesions on T2WI. Wilson disease in a 14-year-old girl with dystonia, with a diagnosis lag time 5 years (participant group >3 years) and abnormal signal in the pons (D), midbrain (E), and frontal and parietal lobes (F) (arrows).
Discussion
Neurologic WD is a genetic disorder that leads to intracellular accumulation of copper in the body, primarily causing damage to the liver and various regions of the brain. Previous studies of MR imaging brain scans in patients with WD have shown abnormal bilateral long T1 and T2 signals in the basal ganglia, especially in the putamen nucleus and head of the caudate nucleus and often in some portion of the thalamus.23 Abnormal bilateral long T1 and T2 signals in the basal ganglia (with or without brain stem impairment) have become one of the important signs of WD.7,23,24 Consistent with the literature, our results also showed long T1 and long T2 abnormal signals in the brain, with the most frequently affected regions including the putamen and caudate nucleus of the basal ganglia (Table 1). The aberrant signal in the basal ganglia is said to be elicited by glial cell hyperplasia as well as edema, necrosis, and lacunae caused by copper deposition.25 However, a study by Prayer et al26 showed that some abnormal signals caused by edema and demyelination can disappear after proper de-coppering therapy. The medulla and occipital lobe were entirely spared, with none of our patients with WD showing abnormalities in these regions (Table 1).
Dystonia is among the most debilitating clinical neurologic symptoms commonly observed in neurologic WD.11,27,28 In our study, the most common areas of brain damage in patients with WD with dystonia were the putamen, pons, and thalamus. Regardless of etiology, symptomatic treatment of dystonia generally depends on its severity and localization.29 According to our findings, dystonia may be related to the putamen, pons, and thalamus.
Similar to dystonia, parkinsonism occurs quite frequently in neurologic WD. Patients with WD show combined presynaptic and postsynaptic nigrostriatal deficits.30 In general neurology, tremor treatment varies depending on the tremor type.31,32 In our patients, the unanticipated finding of corpus callosum abnormalities in the group with parkinsonism resulted in corpus callosum repair in some individuals, with marked improvement of their symptoms. In our study, the most common areas of brain damage in patients with WD with parkinsonism were the putamen, thalamus, and caudate nucleus. Our study findings suggest that parkinsonism may be related to the putamen, thalamus, and caudate nucleus.
Not all of our patients with WD with abnormal caudate nuclei had choreoathetosis. We found that 7 of the 8 patients with WD with choreoathetosis symptoms had an abnormal putamen, and all 8 had an abnormal caudate nucleus. This is basically consistent with the authors' previous research8 indicating that the symptom of choreoathetosis strongly correlates with impairment of the caudate nucleus. Thus, the presence of choreoathetosis predominantly suggests damage in the caudate nucleus.
Torsion spasms are a rare neurologic symptom of WD, which contribute to patient disability when present.7 MR imaging data from our patients showed frequent damage to the midbrain and pons but no damage to the remaining regions of the brain stem and medulla oblongata. Taken together, our MR imaging data show that the putamen is the most frequently damaged brain region in patients with WD, regardless of the accompanying neurologic symptoms, followed by the pons and the thalamus.
The data in Table 2 seem to imply that the disease gradually worsens with age before of 30 years of age, but older age of onset is a mitigating factor in WD; however, we cannot ignore the possibility that the lower frequency of brain stem abnormalities and improved prognosis is owing to some other factor that we did not examine in this study, such as disease duration.
The prognosis of WD is closely related to the diagnosis lag time for each patient.33 As our correlation analysis showed, the frequency of damage observed in the pons, midbrain, and cortex is directly correlated with the diagnosis lag time. Specifically, the longer the period between symptom onset and confirmed diagnosis, the greater the likelihood that the patient will develop impairment in these brain regions. In the present study, the features of WD characterized by torsion spasm included age of onset usually younger than 15 years and diagnosis lag time of at least 2 years (the longest was 23 years). From the trend in the length of diagnosis lag time seen in Table 3, we can speculate that the initial sites of brain injury in neurologic WD are the putamen and thalamus, then developing in the pons and midbrain, and culminating with cortical damage. Therefore, it is crucial for individuals with WD to seek medical care, receive a confirmed diagnosis, and initiate treatment as early as possible.
Differences between our results and those of other MR imaging studies of WD could be due to many reasons: Limited sample sizes, diversity of study participants, variations in the magnetic field strength of the MR imaging scanner used, and duration of therapy all contribute to variations in the results reported in the clinic. The clinical manifestations of WD may be region-specific,16 making the demographic composition of the study cohort an important factor for interpretation of the collected data. With 364 patients with WD recruited only from China and MR images collected before the start of any systemic de-coppering therapy, our study has the crucial advantages of a large sample size focused within a single geographic region and being free from the confounding effects of prior treatment.
This study spans a long time, and MR imaging data from gradient-echo images and diffusion images have only become available in recent years. Therefore, most early studies lack these data, and this feature is a drawback of this study.
Conclusions
Our study findings showed that the putamen, pons, midbrain, and thalamus are highly prone to damage in neurologic WD, whereas the medulla oblongata and occipital lobe seem completely unaffected. The putamen is the most frequently damaged brain region in this study. In patients with age of onset younger than 10 years, cranial MR imaging scans showed impairment only in the putamen. Patients with a longer lag time before diagnosis were more likely to have impairment in the pons, midbrain, and cortex. Among neurologic symptoms of WD, torsion spasm is associated with the midbrain and cortex, and choreoathetosis is primarily related to the caudate nucleus. The brain abnormalities in patients with neurologic Wilson disease are correlated with their clinical symptoms, age of onset, and lag time to diagnosis.
Footnotes
Disclosures: Xu-En Yu—RELATED: Grant: Natural Science Foundation of Anhui Province, Comments: mitochondrial damage mechanism of mouse brain neurons in Wilson disease model TX (grant No. 1508085MH153).* Shan Gao—RELATED: Grant: Natural Science Foundation of Anhui Province, Comments: mitochondrial damage mechanism of mouse brain neurons in Wilson disease model TX (grant No. 1508085MH153).* Ren-Min Yang—RELATED: Grant: Natural Science Foundation of Anhui Province, Comments: mitochondrial damage mechanism of mouse brain neurons in Wilson disease model TX (grant No. 1508085MH153).* Yong-Zhu Han—RELATED: Grant: Natural Science Foundation of Anhui Province, Comments: mitochondrial damage mechanism of mouse brain neurons in Wilson disease model TX (grant No. 1508085MH153).* *Money paid to the institution.
This work was funded by the Natural Science Foundation of Anhui Province (1508085MH153).
Indicates open access to non-subscribers at www.ajnr.org
References
- Received June 26, 2018.
- Accepted after revision October 30, 2018.
- © 2019 by American Journal of Neuroradiology