Abstract
BACKGROUND AND PURPOSE: MR imaging has become an important tool for the detection of cholesteatomas of the middle ear. Various diffusion-weighted imaging sequences are available and have shown promising results. This study aimed to evaluate readout-segmented echo-planar DWI for the detection of cholesteatoma and compare the results with surgical validation.
MATERIALS AND METHODS: Fifty patients with chronic otitis media (24 females and 26 males; range, 12–76 years of age; mean age, 41 years) who underwent MR imaging before an operation of the middle ear (1–169 days) were included. The MR imaging protocol consisted of axial and coronal readout-segmented echo-planar DWI with b-values of 0 and 1000 s/mm2 and 3-mm slice thickness. The readout-segmented echo-planar diffusion-weighted images were fused with standard T2-weighted sequences for better anatomic assignment. The results of the MR imaging evaluation were correlated with the results from the operation.
RESULTs: Readout-segmented echo-planar DWI detected 22 of the 25 cases of surgically proved cholesteatoma. It has an accuracy of 92% (95% confidence interval, 80.8%–97.8%), a sensitivity of 88%, a specificity of 96%, a positive predictive value of 96%, and a negative predictive value of 89%. In 1 case, a positive finding for cholesteatoma with readout-segmented echo-planar DWI could not be proved by histology, and in 3 cases, histology yielded a cholesteatoma that was not detected with MR imaging.
CONCLUSIONS: Readout-segmented echo-planar DWI is a promising and reliable MR imaging sequence for the detection and exclusion of cholesteatoma.
ABBREVIATION:
- RESOLVE
- readout-segmented echo-planar
Cholesteatoma is defined as a mass of keratinizing squamous epithelium in the tympanic cavity, mastoid cells, and the subepithelial connective tissue that can lead to an inflammatory reaction by the progressive accumulation of keratin debris and bone resorption.1⇓–3 Cholesteatoma can only be cured by surgical removal of the entire mass.4 Depending on the surgical technique, the prevalence of residual or recurrent cholesteatoma is as high as 25%.4⇓⇓–7
The detection of cholesteatoma in patients who have undergone a middle ear operation is often difficult due to the grafts used, and regrowing squamous epithelium in the back of the middle ear or mastoid can remain symptomless for a long time. A high-resolution CT scan, which is the basic method for imaging the nonoperated middle ear, cannot reliably distinguish residual or recurrent disease from postoperative changes such as fluid, fibrous tissue, or granulations.8,9 Temporal bone CT scans have low specificity (48%) and sensitivity (43%) for residual or recurrent cholesteatoma.9 Thus, second-look surgery is a standard for the diagnosis of recurrent and residual cholesteatoma. However, it is associated with anesthesia and surgical risks. In approximately one-third of planned second-look procedures, a residual cholesteatoma can be found.10 In well-reconstructed middle ears with normal postoperative clinical findings and good postoperative auditory results, a second-look procedure could be avoided in two-thirds of cases.
As an alternative to second-look surgery, MR imaging has become an important tool for the detection of cholesteatoma of the middle ear. Various DWI sequences are available and have shown considerable improvement in the diagnosis of cholesteatoma, providing a variation of the conventional MR imaging sequences that use the principles of molecular diffusion or Brownian motion to generate contrast. In certain pathologic conditions, the molecular diffusion, which refers to the random movement of water molecules, is restricted. The keratin debris in cholesteatomas restricts water diffusion and produces a high signal intensity. Mucosal edema, fibrosis, and scar or granulation tissue produce a hypointense signal. DWI techniques can be divided basically into EPI-based and non-EPI-based techniques.11 Whereas EPI-DWI consists of single-shot spin-echo pulse sequences, the non-EPI-DWI consists of either single-shot turbo-spin or multishot turbo-spin sequences. Due to different artifacts that can be generated during the acquisition of DWI, such as ghosting, motion, or susceptibility artifacts, non-EPI-DWI is recommended to avoid false-positive results.11,12
Readout-segmented echo-planar (RESOLVE)-DWI is a relatively new alternative technique for obtaining diffusion-weighted images with high quality, delivering sharp images at high spatial resolution and reduced slice thickness. RESOLVE-DWI uses the same diffusion preparation as single-shot EPI. The k-space trajectory is divided into multiple segments in the readout direction, so that the echo spacing is reduced compared with single-shot EPI-DWI; this feature reduces image blurring due to long echo-trains and susceptibility artifacts. Further distortion artifacts are minimized. Usually 2 spin-echoes are acquired to reduce potential phase artifacts, and the second echo is used to generate 2D navigator data for phase correction.11,13
Our purpose was to evaluate RESOLVE-DWI for the detection of cholesteatomas compared with the criterion standard intraoperative and histopathologic findings.
Materials and Methods
Study Overview
In a retrospective study at a single academic center, we analyzed patients with chronic otitis media who had undergone an operation at the Department of Otorhinolaryngology of the Medical University of Innsbruck and underwent MR imaging before the operation from November 2015 to March 2018.
A data base search was initially performed to identify all patients who had undergone a middle ear operation because of chronic otitis media. Only data of patients who underwent MR imaging before the operation were included in the study. Operative reports and histopathologic results were available for all patients. The institutional review board of the Medical University of Innsbruck approved the study (approval number: 1215/2018). Informed consent was not obtained because the data were collected retrospectively and all imaging data were pseudonymized.
Imaging Technique
MR imaging was performed with a 1.5T scanner (Magnetom Avanto-fit; Siemens Healthineers, Erlangen, Germany). The MR imaging protocol consisted of axial and coronal RESOLVE-DWI with b-values of 0 and 1000 s/mm2 and a 3-mm slice thickness (19 acquired slices with each sequence). The acquisition time for each sequence was 3 minutes 1 second. In addition, T2-weighted images in coronal and axial orientations and T1-weighted images with fat saturation in an axial orientation were acquired. RESOLVE–diffusion-weighted images were fused with standard T2-weighted sequences for better anatomic assignment using the software provided by the vendor. DWI was further translated into a color-coded image for better visualization.
MR images were evaluated by 2 experienced radiologists (B.H. with 12 years and M.P. with 8 years of experience in reading head and neck MR images) on the basis of standard diagnostic criteria for cholesteatoma11 with DWI. Examiners were blinded to the results of the operation and histopathology; a final decision on the presence of a cholesteatoma was made in consensus. The main diagnostic criterion for cholesteatoma on DWI is lesion hyperintensity, compared with the signal intensity of brain, on b=0 s/mm2 images that persists or increases on high b-value (800–1000 s/mm2) images.14,15 The so-called “T2 shine through” effect is also observed in cholesteatomas; therefore, ADC values were not integrated into our evaluation.11 Further analysis included reviewing T1-weighted images with fat saturation and T2-weighted images considering known pitfalls (Fig 1).16 The color-encoded images were not included in the evaluation procedure.
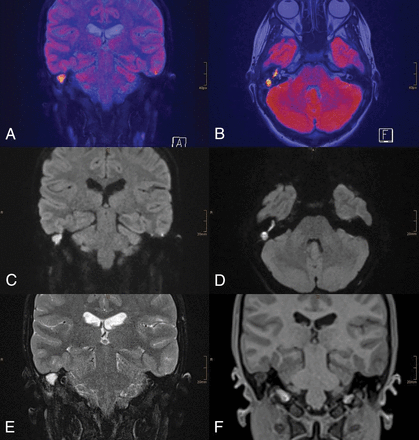
A 13-year-old female patient with chronic otitis media on the right side. RESOLVE-DWI shows a large hyperintense lesion on the right side. A and B, Colored fused images of RESOLVE-DWI (b=1000) and T2-weighted images. C and D, RESOLVE-DWI in coronal and axial orientations (b=1000). T2-weighted image (E) shows a well-delineated T2 hyperintense lesion. T1-weighted image (F) shows no sign of hyperintensity. The cholesteatoma was proved intraoperatively.
Surgical Validation
The diagnosis of cholesteatoma was made on the basis of the intraoperative presence of keratinizing squamous epithelium and debris in the middle ear and/or mastoid and pathohistologic examination of the removed tissue. Microsurgical techniques with the patient under general anesthesia were used in all patients.
Data Analysis
Numeric data were reported as mean ± SD or mean and 95% confidence interval, and categoric data were reported as frequencies and percentages. True-positives, false-positives, true-negatives, and false-negatives were calculated from the findings on RESOLVE-DWI and surgical findings. On the basis of these data, standard diagnostic parameters were calculated. Interobserver agreement of the 2 radiologists' measurements was assessed using the Cohen κ. Statistical analysis was performed using SPSS software, Version 24 (IBM, Armonk, New York).
Results
In this study, 50 MR images (cases) of 47 patients were analyzed. Three patients underwent 2 operations. The patients were 12–76 years of age at the operation (mean age, 41 years). Twenty-four (48%) cases were female, and 26 (52%) cases were male. In 24 (48%) cases, it was the first ear operation, and in 26 (52%) cases, revision surgery was performed. The interval between imaging and the operation was 0–169 days, with a mean interval of 54 days.
The overall rate of interobserver agreement was 92% with a Cohen κ value of 0.84 ± 0.075. In 25/50 cases (50%), a cholesteatoma was detected intraoperatively, and in 22/25, there was a positive finding of surgically validated cholesteatoma on RESOLVE-DWI. In 3/25, there was no hyperintense signal on RESOLVE-DWI despite the surgical and pathohistologic proof of cholesteatoma (Fig 2). In 1/23 cases, a positive finding for cholesteatoma with RESOLVE-DWI could not be proved intraoperatively. RESOLVE-DWI was therefore true-positive in 22/50, true-negative in 24/50, false-positive in 1 case, and false-negative in 3/50 cases. In 46/50 cases (92%), the radiologic and intraoperative evaluations concurred. The sensitivity for detecting cholesteatoma with RESOLVE-DWI was 88% (95% CI, 68.8%–97.5%), the specificity was 96% (95% CI, 79.7%–99.9%), the positive predictive value was 0.96 (95% CI, 0.76–0.99), and the negative predictive value was 0.89 (95% CI, 0.73–0.96). The diagnostic accuracy was 92% (95% CI, 80.8%–97.8%).
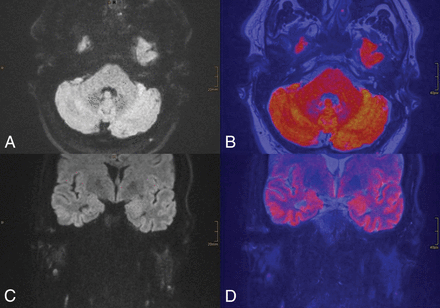
A 52-year-old male patient with suspected cholesteatoma on the right side. MR imaging with RESOLVE-DWI shows no sign of hyperintense signal on the right side (axial and coronal RESOLVE-DWI, A and C, and colored fused images with T2-weighted images, B and D). There were no findings on T1-weighted and T2-weighted images. Intraoperatively, a 4-mm cholesteatoma directly adherent to the malleus was detected. The small size of the lesion probably explained the false-negative results with RESOLVE-DWI.
In 12/24 (50%) patients who had undergone an intervention on the affected ear for the first time, a cholesteatoma was detected intraoperatively. In 10/12 (83%) cases, there was a positive finding of surgically validated cholesteatoma on RESOLVE-DWI. In the 12 surgically negative cases, the RESOLVE-DWI also showed a negative result. The sensitivity for detecting cholesteatoma with RESOLVE-DWI in unoperated ears was 83% (95% CI, 55.2%–95.3%), the specificity was 100% (95% CI, 75.8%–100%), the positive predictive value was 1.00, and the negative predictive value was 0.86 (95% CI, 0.63–0.96).
In 12/26 (46.2%) cases with revision surgery, a cholesteatoma was diagnosed histologically, and in 11/12 (92%) cases, there was a positive finding of surgically validated cholesteatoma on RESOLVE-DWI. In 13/14 (93%) cases in whom no cholesteatoma was found, the MR imaging also showed a negative result. The sensitivity for detecting residual cholesteatoma with RESOLVE-DWI was 92% (95% CI, 64.6%–98.5%), the specificity was 93% (95% CI, 68.5%–98.7%), the positive predictive value was 0.92 (95% CI, 0.63–0.99), and the negative predictive value was 0.93 (95% CI, 0.66–0.99). A summary of the results is shown in the Table.
Accuracy data for the detection of cholesteatomas using MRI with RESOLVE-DWI
Discussion
RESOLVE-DWI is a new technique for the diagnosis of cholesteatoma.11 So far, only a few studies have evaluated this new approach and found promising results.17⇓–19 This study evaluated RESOLVE-DWI for the detection of cholesteatoma and compared the results with surgical validation.
Pooled sensitivity of non-EPI DWI for the detection of residual and recurrent cholesteatomas in a recent meta-analysis was 91% with a specificity of 92%.20 In our study, RESOLVE-DWI reached a sensitivity of 88% and a specificity of 96%. Therefore, in general, both sequences provide comparable results concerning the detection of cholesteatoma. In 1 patient, a positive finding for cholesteatoma with RESOLVE-DWI could not be proved intraoperatively. The reason for the high signal intensity was a wax accumulation in the open mastoid cavity (Fig 3). Lingam et al. also mentioned wax as a reason for false-positive cases: It can produce high signal changes on the b=1000 images and low signal and values on the ADC map.21 Thus, it is important that the open mastoid cavity be cleaned before imaging and the surgeon be aware that wax can lead to a false-positive result.
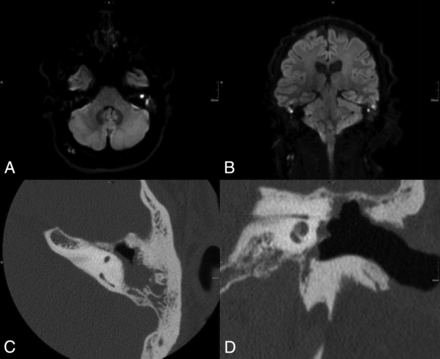
A 31-year-old male patient with suspected recurrent cholesteatoma on both sides. MR imaging with RESOLVE-DWI showed a hyperintense lesion on both sides highly suspicious for cholesteatoma (A and B). The patient underwent an operation of the left ear after the MR imaging. Intraoperatively, there was no sign of recurrent cholesteatoma. The reason for the false-positive MR imaging findings was probably detected wax accumulation in the open mastoid cavity. Axial (C) and coronal (D) CT scans show the radical cavity.
The study by Yamashita et al22 evaluated a multishot EPI sequence for the diagnosis of cholesteatoma and compared the results with a single-shot EPI sequence. They found that multishot EPI improved the accuracy of diagnosis but had no correlation with intraoperative findings or histology. Our RESOLVE sequence differs from the sequence of the Yamashita study group because it is based on a different approach using the same diffusion preparation as single-shot EPI and dividing the k-space trajectory into multiple segments. Potential phase artifacts are reduced by acquiring 2 spin-echoes, with the second echo used to generate 2D navigator data for phase correction. Algin et al17 used a similar approach with a readout-segmented echo-planar imaging–based technique and compared this sequence with single-shot EPI. Compared with our study, the specificity was lower, at only 78% (96% in our study); the sensitivity was slightly higher at 100%. In their study, no correlation with intraoperative findings or histology was available in patients with negative findings on MR imaging. Furthermore, they used 3T, which is, in our opinion, the worst choice for cholesteatomas due to the greater susceptibility at higher field strengths.
In 3 of 25 patients, the surgically validated cholesteatoma could not be detected with RESOLVE-DWI. The images were re-analyzed postoperatively. In 1 case, blood components (due to methemoglobin) in the middle ear, detected with T1-weighted images, caused artifacts, which led to a false-negative result because this was not considered a cholesteatoma. In the other 2 cases, the reason for the false-negative results remained unclear. We assume that these cholesteatomas could not be detected with 3-mm slice thickness RESOLVE-DWI due to their small size.
We did not encounter any relevant artifacts with RESOLVE-DWI that had an influence on radiologic diagnoses in any of our cases. All recognized artifacts could be anatomically clearly assigned to the adjacent brain, which, in turn, was facilitated by the image fusion. Non-EPI-DWI is known to provide less image distortion and artifacts than other DWI techniques.23 Nevertheless, the acquisition time for non-EPI-DWI and EPI-DWI can be quite different. We found acquisition times between 3 and 6 minutes for 1 b-value with non-EPI-DWI in the literature,21 and some studies did not indicate the acquisition time.12,24⇓–26 Therefore, comparison between different sequences is always difficult. As with sequences used in other studies, many parameters such as slice thickness, number of slices, and FOV must be taken into account. Our RESOLVE-DWI takes 3:01 minutes for 1 orientation with 19 slices, 2 b-values, and a slice thickness of 3 mm. In total, our protocol with 2 orientations for RESOLVE-DWI takes <20 minutes, including T1- and T2-weighted sequences, and delivers images with a sufficient SNR ratio. The non-EPI HASTE sequence that is provided by the manufacturer on our scanner takes much longer, with >5 minutes and the same slice thickness and number of images as in our RESOLVE-DWI. The acquisition would also be possible with a smaller number of slices, especially coronal slices. This could further reduce the scanning time.
The limitations of this study include a slice thickness of 3 mm instead of 2 mm, which is already used in some studies. Two-millimeter slices are possible with the RESOLVE-DWI, but keeping the SNR at such a high level would result in a much longer scanning time. Furthermore, we performed no direct comparison with other DWI techniques such as non-EPI-DWI, but this should definitely be considered for future studies with emphasis on a correlation with histopathology. Because of the retrospective character of our study, the size of the intraoperatively found cholesteatomas was not documented and could not be further evaluated. This feature could have explained the false-negative results.
The results of this study support RESOLVE-DWI having high sensitivity and specificity for detecting cholesteatomas. Sinus tympani disease and incus erosion are associated with higher rates of cholesteatoma recurrence; thus, in these cases, a second-look operation should be considered.27 In asymptomatic patients with normal postoperative clinical findings and good hearing results, a postoperative follow-up with RESOLVE-DWI can be recommended to reduce the number of avoidable second-look procedures.28 RESOLVE-DWI remains a promising alternative to non-EPI-DWI.
Conclusions
RESOLVE-DWI is highly sensitive and specific in identifying residual and recurrent cholesteatomas.
Footnotes
Disclosures: All authors declare that there are no conflict of interest related to the subject matter or material discussed in this article.
REFERENCES
- Received December 6, 2018.
- Accepted after revision April 21, 2019.
- © 2019 by American Journal of Neuroradiology