Abstract
SUMMARY: Coronavirus disease 2019 (COVID-19) is a serious public health crisis and can have neurologic manifestations. This is a retrospective observational case series performed March 1–31, 2020, at New York University Langone Medical Center campuses. Clinical and imaging data were extracted, reviewed, and analyzed. Two hundred forty-two patients with COVID-19 underwent CT or MRI of the brain within 2 weeks after the positive result of viral testing (mean age, 68.7 ± 16.5 years; 150 men/92 women [62.0%/38.0%]). The 3 most common indications for imaging were altered mental status (42.1%), syncope/fall (32.6%), and focal neurologic deficit (12.4%). The most common imaging findings were nonspecific white matter microangiopathy (134/55.4%), chronic infarct (47/19.4%), acute or subacute ischemic infarct (13/5.4%), and acute hemorrhage (11/4.5%). No patients imaged for altered mental status demonstrated acute ischemic infarct or acute hemorrhage. White matter microangiopathy was associated with higher 2-week mortality (P < .001). Our data suggest that in the absence of a focal neurologic deficit, brain imaging in patients with early COVID-19 with altered mental status may not be revealing.
ABBREVIATIONS:
- CoV
- coronavirus
- COVID-19
- coronavirus disease 2019
- SARS-COV-2
- Severe Acute Respiratory Syndrome coronavirus 2
The novel coronavirus (CoV), responsible for the December 2019 outbreak in Wuhan, China, has spread quickly around the world, leading to a global pandemic.1 The virus shows similarities in cellular receptors and symptoms to Severe Acute Respiratory Syndrome CoV and has been named Severe Acute Respiratory Syndrome coronavirus 2 (SARS-COV-2), with the disease it causes called coronavirus disease 2019 (COVID-19).
As of April 25, 2020, there were 925,758 confirmed cases of COVID-19 and 52,217 total deaths in the United States.2 The New York metropolitan area has become the epicenter of COVID-19 in the United States. As of April 24, 2020, the City of New York has had 146,139 confirmed cases of COVID-19 and 10,746 confirmed deaths due to the disease.3
A variety of neurologic manifestations have been reported in COVID-19, affecting as many as 36.4% of patients according to a report from Wuhan, China.4,5 Clinically most important, acute ischemic infarcts and intracranial hemorrhage have been noted in these patients.6
Considering that altered mental status in patients with COVID-19 is common secondary to respiratory distress and hypoxemia, brain imaging is frequently considered. Anecdotally, it has been suggested that patients with COVID-19 are at specific risk for ischemic and hemorrhagic central nervous system complications.7 Here, we report the use and findings of neurologic imaging in patients with COVID-19 during the initial month after the outbreak in the City of New York and analyze neuroimaging use as well as acute intracranial findings, including acute infarcts and intracranial hemorrhage.
MATERIALS AND METHODS
This is a retrospective and Health Insurance Portability and Accountability Act–compliant study that was performed following the approval by the institutional review board. Informed consent was waived.
Patients seen at New York University Langone Medical Center (across the Manhattan and Brooklyn campuses) with a positive polymerase chain reaction from a nasal swab specimen for SARS-COV-2 diagnosed between March 1 and 31, 2020, with at least 1 brain imaging examination (CT or MRI) during the course of their recent hospital encounter and within 2 weeks following the positive result of viral testing were included. Electronic health records and neuroimaging studies were reviewed for age, sex, patient type (outpatient or inpatient status), clinical indication for brain imaging (from clinical notes), imaging findings, and 2-week outcome (mortality, transition to hospice or comfort care, improved or stable clinical condition). For patients with imaging findings of acute or subacute infarcts, note was made of anterior-versus-posterior vascular territory involvement, small- or large-vessel occlusion infarct, the presence or absence of hemorrhagic transformation, and whether revascularization with mechanical thrombectomy was attempted, as well as the imaging outcome of revascularization. In cases of white matter microangiopathy, a neuroradiologist with 6 years of subspecialty experience graded microangiopathies as none, as much as expected for age or more than expected for age on CT and MRI.8 Mean ages for different categoric variables were compared using a 2-tailed t test. The difference between ratios for categoric variables was examined using Fisher exact tests (QuickCalcs; www.graphpad.com/quickcalcs/).
RESULTS
Imaging Use and Common Findings
Of 3661 patients with laboratory-confirmed diagnosis of SARS-COV-2 between March 1 and 31, 2020, two hundred forty-two patients underwent at least 1 cross-sectional brain imaging examination. These 242 patients had a mean age of 68.7 ± 16.5 years; there were 150 men (62.0%) and 92 women (38.0%), 231 inpatients and 11 outpatients. The patients from the emergency department were considered outpatients if they were discharged home from the emergency department and were considered inpatients if they were transferred from the emergency department to the intensive care unit or regular floors of the hospital. Two hundred 7 patients had only CT, 11 patients had only MRI, and 24 patients underwent both CT and MRI.
Overall, the most common abnormal findings seen on imaging were nonspecific white matter changes (hypodensity on CT or T2 hyperintensity on MRI, often attributed to microangiopathy) in 134 patients (55.4%), followed by chronic infarct in 47 patients (19.4%), acute or subacute infarcts in 13 patients (5.4%), and acute intracranial hemorrhage in 11 patients (4.5%). White matter microangiopathy was as much as expected for age in 108 and more than expected for age in 26 patients. One patient had imaging findings of widespread anoxic brain injury following a large acute supra- and inftratentorial hemorrhage (Fig 1A).
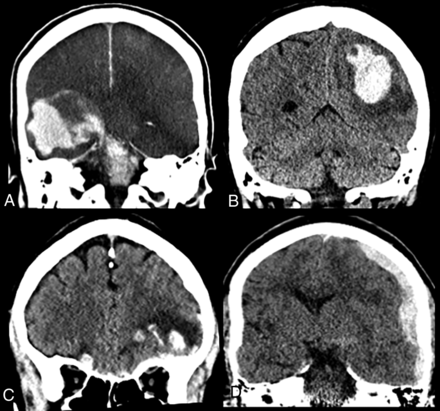
Acute intracranial hemorrhage in patients with COVID-19. A, A 74-year-old man with COVID-19, intubated for hypoxic respiratory failure and on heparin due to a history of stented carotid stenosis. On day 10 of intensive care unit admission, he suddenly became unresponsive, and neurologic examination revealed absent brain stem reflexes. Brain CT showed extensive supra- and infratentorial acute hemorrhage with subarachnoid and intraventricular extensions, along with cerebral swelling and hypodensity (likely hypoperfusion injury), as well as uncal, subfalcine, and transtentorial herniations. B, A 61-year-old woman with COVID-19 and compensated hepatic cirrhosis (due to primary sclerosing cholangitis). On day 7 of intensive care unit admission, the patient developed right-sided weakness and numbness. Brain CT showed left parietal intraparenchymal hemorrhage with surrounding vasogenic edema. C, A 68-year-old man with COVID-19 was found fallen. Head CT showed bilateral inferior frontal lobe hemorrhagic contusions and a small subarachnoid hemorrhage, suggesting traumatic brain injury, likely related to the fall. D, A 61-year-old man with COVID-19 who presented after a fall. Brain CT revealed acute left cerebral convexity subdural and left ambient cistern subarachnoid hemorrhage, possibly related to the fall.
The 3 most common clinical indications for brain imaging were the following: 1) altered mental status (102 patients, 42.1%, all were inpatients), 2) syncope/fall (79 patients, 32.6%, including 4 outpatients), and 3) focal neurologic deficit (30 patients, 12.4%, all were inpatients). Of note, 5 outpatients were imaged for nonacute headache, and 2 were imaged for generalized weakness. Of patients imaged for altered mental status, 42 (41.2%) had white matter microangiopathic changes, 29 (28.4%) had chronic infarcts, and 1 patient had an incidental meningioma. No patients with altered mental status as the indication for brain imaging demonstrated acute or subacute infarct or acute intracranial hemorrhage.
In a 2-week follow-up period, 63 patients died or were transitioned to hospice or comfort care and 179 showed improvement or stability. The mean age of patients with fatal outcome (76.4 ± 13.1 years) was significantly higher than that of the patients who remained stable or clinically improved during the subsequent 2 weeks (66.0 ± 16.8 years) (P value < .001). White matter microangiopathy on brain imaging showed an association with poor 2-week outcome (P < .001). Two-week outcome was not significantly different between men and women (P = .88).
Acute/Subacute Infarcts
Acute and subacute infarcts were categorized together because the distinction is often difficult on CT, which composed most of our examinations. In the 13 patients with acute/subacute infarct, 11 (84.6%) had a focal neurologic deficit as the primary indication for brain imaging and the remaining 2 patients were imaged for syncope/fall. Based on the Fisher exact test for comparing ratios, there was a highly statistically significant association between focal neurologic deficit and the presence of acute/subacute infarct (P < .001). Of note, none of 102 patients scanned for altered mental status alone were subsequently demonstrated to have acute or subacute infarct. In addition, 19 patients of a total of 29 with focal neurologic deficits (65.5%) showed no acute or subacute infarcts on CT or MRI.
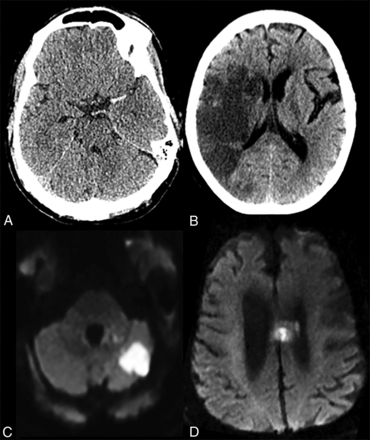
In these 13 patients with acute or subacute infarct, 9 infarcts (69.2%) involved the anterior and 4 (30.8%) involved the posterior circulation territories. Four patients had large-vessel occlusions, including occlusion of the distal left ICA, proximal M1 segment of the left MCA (Fig 2A), right M2 segment (Fig 2B), and the right posterior cerebral artery. All 3 patients with anterior circulation large-vessel occlusions underwent mechanical thrombectomy with TICI 3, 2a, and 2b revascularizations, respectively.
Acute or subacute infarct in patients with COVID-19. A, A 62-year-old man with COVID-19, intubated for acute hypoxic respiratory failure, who initially presented with left MCA syndrome. Noncontrast head CT showed a dense left MCA sign suggesting a left MCA occlusion, later confirmed on CT angiography and catheter angiography (not shown here). B, A 77-year-old woman with COVID-19 presented with left-sided weakness. Noncontrast CT showed an acute/subacute right MCA territory infarct. C, A 63-year-old COVID-19 patient with ataxia. Brain MRI revealed a patchy acute infarct in the left cerebellar hemisphere. D, A 78-year-old man with COVID-19 presented following an unwitnessed fall. Brain MRI showed left more than right cingulate gyrus and callosal body acute infarct.
In 6 of 13 patients with acute or subacute infarcts, the infarcts were noted at presentation, and in 7 patients, infarcts were detected during the hospitalization. Only 1 patient with acute or subacute infarct showed hemorrhagic transformation in the infarcted region after receiving intravenous tPA. Two other patients who received tPA did not show any complications. Three of 13 patients with acute or subacute infarcts also showed chronic infarcts on brain imaging. While 11 of 13 patients had some degree of white matter microangiopathy on brain imaging, none was perceived to be more than expected for age.
A statistically greater number of patients with acute/subacute infarcts on neuroimaging had white matter microangiopathic changes compared with patients without infarct (11 of 13 compared with 123 of 229, P = .04). On the other hand, no statistically significant difference was seen between patients with and without acute/subacute infarct in terms of age (mean, 69.08 ± 12.5 years versus 68.69 ± 16.77 years, respectively, P = .93) or the presence of chronic infarct on brain imaging (10 of 13 versus 185 of 229, P = .72).
Acute Hemorrhage
Eleven patients demonstrated areas of acute hemorrhage on brain CT or MRI. In 4 of these patients, review of history and prior imaging showed the hemorrhage to be present 4–8 weeks prior to the current hospital encounter and related to an automobile crash, tumor resection, shunt placement, and hypertensive hemorrhage. These cases were thus considered unrelated to COVID-19. Of the remaining 7 patients who were all hospitalized, 4 were found fallen or brought to the hospital after a fall with a subsequent positive test for SARS-COV-2. All of these 4 patients also had respiratory distress and/or hypoxemia at presentation, presumably related to COVID-19. The causal relationship between the fall and COVID-19 is unclear. Two patients had dramatic intraparenchymal hemorrhage during the hospitalization (Fig 1A, -B), and both died. One remaining patient had a fall, and subsequent imaging revealed scattered convexity subarachnoid hemorrhage, believed to be posttraumatic, as well as a patchy area of acute infarct in the cingulate gyrus (Fig 2D). Among these 7 patients with acute intracranial hemorrhage, 4 hemorrhages were extra-axial, 1 was intra-axial, and 2 were mixed, with 6 being supratentorial and one being both supra- and infratentorial (Fig 1A).
Among these 7 patients with acute hemorrhage, the clinical indication for imaging was syncope/fall in 4 patients and focal neurologic deficit in 3 patients. None of 102 patients who were imaged for altered mental status had acute intracranial hemorrhage.
DISCUSSION
Patients with COVID-19 may undergo brain imaging for a variety of clinical reasons. In a recently published series by Mao et al4 from Wuhan, China, 36.4% of patients with COVID-19 were reported to have neurologic manifestations, including 28.2% with altered mental status or acute cerebrovascular disease.4 We report on 242 of 3661 patients with COVID-19 (6.6%) who underwent brain imaging. The discrepancy between rates likely relates to multiple factors: Mao et al reported all perceived neurologic symptoms including nonspecific symptoms such as headaches or dizziness, whereas here we report neurologic symptoms specifically leading to imaging. In addition, there are likely to be individuals in our overall cohort who were too ill for imaging, though they may have had neurologic manifestations of disease.
While altered mental status was the most common clinical reason for brain imaging, no acute/subacute infarct or acute hemorrhage was identified in any patient imaged for altered mental status alone within the first 2 weeks after positive result of viral testing. On the other hand, the overwhelming majority of patients with imaging findings of acute/subacute infarct had focal neurologic deficits on clinical examination. Similarly, patients with acute hemorrhage had either focal neurologic deficits or a history of syncope or fall. Severe acute respiratory syndrome and hypoxemia that can be seen in COVID-19 can lead to altered mental status. While alteration in mental status warrants a complete clinical neurologic examination, if a trusted clinical examination fails to elicit a focal neurologic deficit and there is no history of syncope or fall, brain imaging may not be particularly revealing, an observation that is concordant with prior studies of brain imaging in acute altered mental status.9
Risks and benefits of imaging should certainly be considered in these patients who are highly contagious, with the potential risk of exposure to staff and other patients in the process of transport and imaging. On the other hand, the presence of a focal neurologic deficit, a recent episode of syncope or fall, or lack of a reliable neurologic examination appear to be good reasons to pursue neuroimaging in a neurologically symptomatic patient. We would like to caution the readers about interpreting this observation and applying it to their practice, particularly in view of the excessive use of the term “altered mental status” as a clinical indication. In fact, the American College of Radiology Appropriateness Criteria support brain imaging for the evaluation of patients with altered mental status in most clinical scenarios. Therefore, we suggest that when the clinical indication for brain imaging of patients with COVID-19 is altered mental status, the radiologist should consider reviewing the electronic health record in more detail or discuss the risks and benefits with the requesting physician in order to ensure that the benefits of such examination outweigh the risks in the setting of a viral outbreak.10
The reported prevalence of stroke in the adult population in the United States is 3%.11 Indeed, hospitalization for acute infection is associated with a transient increase in the risk of vascular events including stroke, with an odds ratio of 8.0 for 14 days preceding the stroke.12,13 Previous studies have specifically reported acute cerebrovascular disease in COVID-196 and cited thromboembolic predisposition7 and increased blood viscosity secondary to the virus attacking the β chain of hemoglobin and causing hypoxemia.14 In our study, the overall rate of an acute/subacute infarction or acute hemorrhage is 8.3%. This may well be an under-representation of the true incidence of acute cerebrovascular events in these patients because some may have been too sick or declined too rapidly to warrant imaging.
Among those patients who were imaged, we found that 5.4% had acute/subacute infarcts. This is higher than the 4.2% incidence of stroke among the hospitalized patients with neuroimaging at our institution during March 2019 (personal communication, Dr Eytan Raz, New York University Langone Medical Center, April 19, 2020). The reason for the noted difference is likely multifactorial. In addition to possibly higher rates of infarct among patients with COVID-19 compared with hospitalized patients without COVID-19, this difference could be related to the less sick patients avoiding hospitals or emergency departments during the COVID-19 outbreak and resulting in an overall increased rate of presentation of more advanced stages of disease, or be related to primary teams requesting neuroimaging examinations more selectively during the outbreak in view of the limited resources.
Acute intracranial hemorrhage in patients with COVID-19 may result from fall/syncope15 or could occur without a preceding mechanical trauma. Previously reported acute hemorrhagic necrotizing encephalopathy following COVID-1916 was not seen in any of our patients, suggesting a low incidence of such findings.
Higher mortality was seen in patients with white matter microangiopathy compared with those without. This likely relates to a known association of these imaging changes with age and cardiovascular risk factors, which have both been associated with poor prognoses in patients with COVID-19. In the absence of a reliable medical history for these patients, microangiopathic changes can serve as a window to the patient’s long-standing underlying risk factors17 and can provide potentially prognostic insights.
Limitations of the current study include variability in the imaging modality: CT and MRI findings were studied together and most (85.5%) patients had only CT. The high proportion of CT may result in under-reporting of acute imaging findings because the sensitivity of MRI is known to be higher. In many cases, causal and even temporal relationships between COVID-19 and the patient’s neurologic events are difficult to ascertain; therefore, any conclusion with potential management implications must be confirmed with future investigations. Future prospective studies would help elucidate and pave the way toward more concrete guidelines. As the City of New York marches toward what we hope to be the downslope of the current outbreak, these data may help radiologists and neurologists across regions that are peaking at a later time and provide potential insight in case of future respiratory viral outbreaks.
Footnotes
Disclosures: Alireza Radmanesh—UNRELATED: Other: retirement accounts, with possible investments in biomedical companies; none would impact the content of this work. Eytan Raz—UNRELATED: Expert Testimony: various law firms; Royalties: Springer; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: MicroVention. Elcin Zan—OTHER RELATIONSHIPS: Consulting Fee or Honorarium, speaker honorarium but nothing relevant to the topic of this article: Advanced Accelerator Applications, a Novartis company.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received April 25, 2020.
- Accepted after revision May 3, 2020.
- © 2020 by American Journal of Neuroradiology