Abstract
BACKGROUND AND PURPOSE: The treatment paradigm for very large and giant aneurysms has recently changed to flow diversion, in light of the results of the Pipeline for Uncoilable or Failed Aneurysms trial. However, the effects of flow diversion were definitely unknown. We explored this topic and identified the predictors of such effects.
MATERIALS AND METHODS: We retrospectively reviewed 51 patients with unruptured aneurysms admitted to our institution for flow diversion between February 2014 and August 2019. Patients were categorized into an effect group (no filling or remnant entry) and a no-effect group (subtotal or total filling). We evaluated the aneurysm size and shape, incorporation vessel, parent artery stenosis and curvature, stagnation of contrast medium within the aneurysm, use of balloon angioplasty, and intra-aneurysm thrombus as potential predictors of the effects of flow diversion.
RESULTS: The effect group comprised 34 patients (66.7%, 34/51; no filling, 35.3%, 18/51; and remnant entry, 31.4%, 16/51). The no-effect group comprised 17 patients (33.3%, 17/51; subtotal filling, 29.4%, 15/51; and total filling, 3.9%, 2/51). An incorporation vessel and balloon angioplasty were independent risk factors for the no-effect group in multivariate logistic regression analyses (OR = 0.13 and 0.05; 95% confidence intervals, 0.02–0.62 and 0.00–0.32; P values, .021 and .004, respectively).
CONCLUSIONS: Flow diversion is effective for very large and giant aneurysms, but the outcomes require further improvement. The results of this study show that an incorporated vessel and excessive balloon angioplasty might compromise flow diversion. This finding can help improve the outcomes of flow diversion.
ABBREVIATIONS:
- FD
- flow diversion
- FDs
- flow-diverter stent
Very large (15–25 mm) and giant (>25 mm) cerebral aneurysms are at high risk for fatal rupture.1,2 Conventional surgical treatment is associated with a low rate of complete ligation and a high incidence of surgical complications.3,4 Given the development of new endovascular techniques and devices and the results of the International Subarachnoid Aneurysm Trial (ISAT),5 coil embolization is now the preferred treatment for many kinds of cerebral aneurysms. However, many studies reported that coil embolization for very large and giant aneurysms has a high recurrence rate.6⇓-8 Thus, considering the results of the Pipeline for Uncoilable or Failed Aneurysms (PUFS) trial,9 the preferred treatment has recently been changed to flow diversion (FD). However, the effects of FD remain definitely unknown. We explored this topic and identified the predictors of the effects of FD.
MATERIALS AND METHODS
Patient Selection
We retrospectively reviewed the records of patients with unruptured aneurysms consecutively admitted to our institution for FD by a single surgeon (Y.S. Shin) from February 2014 to August 2019. According to the approval criteria of the Korean government (Health Insurance Review and Assessment Service) for flow-diverter stents, these were placed in patients with unruptured intracranial aneurysms of ≥15 mm in maximum diameter without coil embolization. A total of 64 patients underwent FD; catheter-based angiographic follow-up data were available for 51 patients. We retrospectively analyzed their outcomes and sought their predictors. The study was approved by the institutional review board of Seoul St. Mary's Hospital.
Baseline and Follow-up Assessments
The records included patient sex and age; the presence of diabetes mellitus, hypertension, and hyperlipidemia; current smoking status; aneurysm size, location, neck diameter, and shape (saccular or fusiform); intra-aneurysm thrombus and incorporated vessel status; stenosis status and curvature of the parent artery in the aneurysm neck region; contrast medium stagnation during angiography; balloon angioplasty in the procedure; and angiographic and clinical outcomes. All angiographic outcomes were blindly evaluated by 1 neurointerventionalist and 1 neuroradiologist using the O'Kelly-Marotta scale based on the degree of filling in catheter-based angiographic follow-up (no filling, remnant entry, subtotal filling, and total filling).10 MR angiography and catheter-based angiography were performed 6 months after treatment. If incomplete occlusion was evident in the first catheter-based angiography, follow-up was performed at 12 and 24 months. Patients were categorized into an effect group (no filling or remnant entry) and a no-effect group (subtotal or total filling) based on the catheter-based angiographic data. The 5-year results of the PUFS trial11 indicated that aneurysms evidencing remnant entry were likely to completely occlude across time without further treatment.
Predictors of the Effects of FD
The parameters evaluated were aneurysm shape (saccular or fusiform), size, and neck diameter; intra-aneurysmal thrombus and incorporated vessel status; stenosis and curvature of the parent artery in the region of the aneurysm neck; balloon angioplasty status; and contrast medium stagnation during angiography as potential predictors of the effects of FD.9,12 The curvature of the parent artery involving the aneurysm neck region was classified as outer or non-outer, depending on the location of the aneurysm neck (Fig 1). Because the pore density of flow-diverter stents is greatly affected by curvature, metal coverage is relatively higher over the inner curve than over the outer curve.13 If the aneurysm neck lies on the outer curve, any effects of flow-diverter stents are likely to be reduced. The stagnation grade of contrast medium was categorized as arterial, capillary, or venous on pre- and post-FD cerebral angiography. Immediately after flow-diverter stent (FDs) deployment, it was determined whether the stagnation grade increased. In addition, prolonged stagnation (for >1 minute) was recorded after FDs deployment. We assume that balloon angioplasty changes the stent pore density because the flow-diverter stents are braided and the aneurysm neck lies in the unconstrained zone.
This image shows the curvature type of the parent artery involving the neck region of the aneurysm. A, Non-outer type. B, Outer type.
Procedure
All procedures were performed with the patient under general anesthesia following systemic intravenous heparinization and premedication with antiplatelet drugs (aspirin, 100 mg, and clopidogrel, 75 mg, daily for at least 7 days). After the dual-antiplatelet therapy for 6 months, only aspirin, 100 mg, was maintained continuously. The platelet function test was routinely performed on the day of the procedure. The antiplatelet medication was not modified in patients with resistance because our other study showed that antiplatelet drug resistance did not increase the thromboembolic events after stent-assisted coiling.14 Because we encountered delayed rupture of aneurysms treated via FD, dexamethasone was prescribed for 3 weeks after FDs deployment as prophylaxis for intradural aneurysms. The Pipeline Embolization Device (PED; Medtronic) and the Flow-Redirection Endoluminal Device (FRED; MicroVention) were used for flow-diverter stents. When parent artery stenosis or poor stent apposition to the vessel wall was evident in conebeam CT performed immediately after FDs deployment, balloon angioplasty was performed using the Scepter device (MicroVention) along with the FDs.
Statistical Analyses
Categoric and continuous variables are reported as means (SD) and ranges and as frequencies with percentages, respectively. Demographic, clinical, and radiologic variables were compared between the 2 groups using the Student t test, the Mann-Whitney U test, the Fisher exact test, or the χ2 test, as appropriate. Predictors with P < .20 in univariate analyses were included in the multivariate logistic regression model in a backward stepwise method to identify the effects of FD.12 All data were analyzed using R statistical and computing software, Version 3.3.2 (https://www.r-project.org/).
RESULTS
The clinical characteristics of the 51 patients who underwent follow-up angiography after FD are summarized in the Online Supplemental Data. There were 34 patients in the effect group (66.7%, 34/51; no filling, 35.3%, 18/51; remnant entry, 31.4%, 16/5). The no-effect group comprised 17 patients (33.3%, 17/51; subtotal filling, 29.4%, 15/51; total filling, 3.9% 2/51). The mean age of the effect group was lower than that of the no-effect group (51.2 [SD, 14.9] years versus 57.8 [SD, 12.8] years; P = .126). The mean aneurysm size and neck diameter were 21.9 (SD, 4.3) mm and 10.1 (SD, 4.8) mm, respectively (effect group: 21.4 [SD, 4.1] mm, 9.8 [SD, 4.4] mm; no-effect group: 25.4 [SD, 12.7] mm, 13.2 [SD, 15.4] mm; P = .231, P = .385, respectively). The mean aneurysm size and neck diameters were larger in the no-effect group, but these differences did not reach statistical significance. The effect group was observed in 11/19 (57.8%) aneurysms involving the infraclinoid internal carotid artery, 14/20 (70.0%) aneurysms involving the supraclinoid internal carotid artery, 3/4 (75.0%) aneurysms involving the anterior and middle cerebral arteries, and 6/8 (75.0%) aneurysms involving the vertebrobasilar artery. The factors such as aneurysm size, vessel incorporation into the aneurysm, aneurysm neck located on an outer curve of the parent artery, and intraprocedural balloon angioplasty yielded P values <.200 in univariate analyses; thus, these were included as predictors of the effect in multivariate analyses (Table 1). Vessel incorporation into the aneurysm and intraprocedural balloon angioplasty (OR = 0.13 and 0.05; 95% CI, 0.02–0.62 and 0.00–0.32; P value, .021 and .004, respectively) were significant risk factors for the no-effect group in multivariate logistic regression analyses (Table 2). Among all 51 patients, complications occurred in 7, including 4 deaths (3 delayed ruptures and 1 thromboembolic event) and 3 major complications (1 each of rupture, thromboembolic event, and aneurysm mass effect). The overall morbidity and mortality rates were 3.9% (2/51) and 7.8% (4/51), respectively.
Results of univariate analysis for predictors of effect group
Results of multivariate logistic regression analysis for predictors of effect group
DISCUSSION
Despite recent improvement in endovascular devices, the treatment outcomes of very large and giant aneurysms remain unsatisfactory.6,7,15,16 Sluzewski et al16 reported a 41% (12/29) incomplete occlusion rate after initial and repeat coiling for very large and giant aneurysms. In addition, recurrence after coil embolization for very large and giant aneurysms was more common than after coil embolization for simple aneurysms. Chalouhi et al15 reported recurrence and retreatment rates of 46.8% (29/62) and 37.1% (23/62) for very large aneurysms and 52% (11/21) and 47.6% (10/21) for giant aneurysms, respectively, after coil embolization. After the PUFS trial, the introduction of FD induced a paradigm shift in endovascular treatment of such aneurysms.11,17⇓-19 For large and giant aneurysms after FD, Peschillo et al17 and Oishi et al19 reported 61.5% (16/26) and 63% (63/100) no-filling rates, respectively. In our study, the no-filling and remnant entry rates were 35.3% (18/51) and 31.4% (16/51), respectively. When we considered remnant entry to be equivalent to no filling, our occlusion rate was similar to those reported in other studies.17,19
However, the actual no-filling rate in this study was lower than those reported in other studies9,12 for several reasons: First, the size of the aneurysms in our study was at least 15 mm, and the mean aneurysm size was 21.9 (SD, 4.3) mm, which is larger than the 18.2 mm reported in the PUFS trial. To the best of our knowledge, this is the largest average aneurysm size reported in any study to date. The aneurysm size is known to affect FD.12,18,20⇓-22 Second, only a single FDs was placed in all but 5 patients (in whom a single FDs could not cover the aneurysm neck or it was foreshortened) because the Korean National Insurance scheme does not usually permit using multiple flow-diverter stents or combined with coil embolization.23 Other studies that used multiple flow-diverter stents or coiling combined with FD have reported that these approaches are better than the placement of a single FDs.17,24⇓-26 In addition, other studies with the same treatment inclusion criteria as ours showed similar results. The authors reported an approximately 77% complete or near-complete occlusion rate in a multicenter study.27 Third, the patient inclusion criteria in the PUFS trial were aneurysms involving the internal carotid artery from the petrous segment to the supraclinoid segment. In our study, there were 14/51 (27.4%) aneurysm cases (posterior circulation, 8 cases; internal carotid artery bifurcation, 2; middle cerebral artery, 3; anterior communicating artery, 1) that did not correspond to the PUFS trial. These 14 cases may have more incorporated vessels that were or were not angiographically visible. However, there is a lack of studies analyzing FD outcomes based on the location of aneurysm. Fourth, our study reported short-term results (follow-up duration, 83.0 [SD, 60.5] weeks). The results of the 5-year PUFS trial suggest that the aneurysm healing process following FD occurs progressively. The trial reported that 7 of the 9 remnants identified were occluded on subsequent angiographic studies without retreatment.11 Therefore, we considered remnant entry as the effect group.
In this study, it was confirmed that the presence of incorporated vessels (OR = 0.13, P = .021) was a significant factor in predicting the effects of FD. Among cases with no incorporated vessel, 22/29 (75.8%) showed the effect of FD, whereas in cases with incorporation, only 12/22 (54.6%) cases showed the effect of FD. In patients exhibiting remnant entry or subtotal filling on follow-up angiography, a significant proportion of aneurysms developed thromboses and occlusion, but sometimes remnant aneurysms were apparent close to the incorporated vessels (Fig 2). Bender et al12 studied a single-institution series of 445 cases and reported that vessel incorporation was a risk factor for incomplete occlusion after FD (OR = 2.206, P = .035).
Remnant aneurysms are apparent close to the incorporated ophthalmic artery on 6-month follow-up angiography.
In-stent balloon angioplasty is performed when the stent-to-wall apposition is poor or stenosis is evident in the parent artery because either condition greatly increases the risk of thromboembolic events and subsequent parent artery occlusion.18,28,29 In addition, high-velocity blood flow into the aneurysm caused by stenosis of the proximal parent artery compromises the effect of FD and might induce delayed aneurysm rupture (Fig 3).20,30,31 However, we found that patients who underwent in-stent balloon angioplasty are more likely to be in the no-effect group (OR = 0.05, P = .004). Of the no-effect group, 6/17 (35.3%) patients underwent in-stent balloon angioplasty, while 3/34 (8.8%) in the effect group underwent angioplasty. To the best of our knowledge, no other study has shown that balloon angioplasty compromises the effect of FD. Because FD efficacy is greatly affected by pore density, interventionists sought to maximize this density using the push-and-pull technique at the unconstrained area of the parent artery during FDs deployment. This balloon angioplasty might increase the pore density in the non-neck area and decrease it in the neck area. However, stenosis of the parent artery confounds the effects of balloon angioplasty on the FDs. As mentioned above, angioplasty was performed when stenosis was apparent. Such stenosis of the parent artery is more likely to occur owing to the mass effect than the size of the aneurysm. However, there was no significant trend toward increased aneurysm size in parent artery stenosis in our study (effect group, 21.9 mm [SD, 8.6 mm] and no-effect group, 25.9 mm [5.4 mm], P = .151). In addition, this information should be interpreted with caution, given the low incidence of balloon angioplasty. Many studies have found that balloon angioplasty is required during FD. Our findings imply that excessive balloon angioplasty, which is performed along the whole stent or when there is a slight suspicion of poor stent-to-wall apposition, should be avoided.
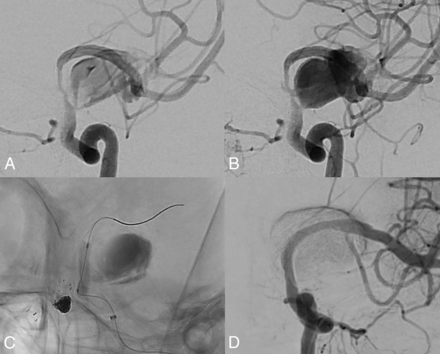
High-velocity blood flow into the aneurysm was caused by proximal parent artery stenosis after flow diversion. Early artery phase (A) and late artery phase (B). The high-velocity blood flow disappears immediately (D) after balloon angioplasty in the stent (C).
Limitations
The present study has several limitations. First, this is a retrospective study of a relatively small number of cases in a single center. Follow-up angiography was not completed in all cases, and the follow-up duration was not long enough. Therefore, the inability to obtain statistically significant results for all data analyzed is a limitation of this study. Second, the self-adjudication of aneurysm occlusion is known to be considerably overestimated according to another study.32 However, we treated only large and giant aneurysms, so there was relatively little difference in distinguishing the effect group from the no-effect group according to the O'Kelly-Marotta grading scale criteria.
CONCLUSIONS
FD is effective for treating very large and giant aneurysms, but the outcomes require further improvement. The results of this study show that an incorporated vessel and excessive balloon angioplasty might compromise the effect of FD. Considering this finding can help improve the outcomes of FD.
References
- Received August 18, 2020.
- Accepted after revision December 24, 2020.
- © 2021 by American Journal of Neuroradiology