Abstract
BACKGROUND AND PURPOSE: FLAIR vascular hyperintensities are thought to represent leptomeningeal collaterals in acute ischemic stroke. However, whether all–FLAIR vascular hyperintensities or FLAIR vascular hyperintensities–DWI mismatch, ie, FLAIR vascular hyperintensities beyond the DWI lesion, best reflects collaterals remains debated. We aimed to compare the value of FLAIR vascular hyperintensities–DWI mismatch versus all–FLAIR vascular hyperintensities for collateral assessment using PWI-derived collateral flow maps as a reference.
MATERIALS AND METHODS: We retrospectively reviewed the registries of 6 large stroke centers and included all patients with acute stroke with anterior circulation large-vessel occlusion who underwent MR imaging with PWI before thrombectomy. Collateral status was graded from 1 to 4 on PWI-derived collateral flow maps and dichotomized into good (grades 3–4) and poor (grades 1–2). The extent of all–FLAIR vascular hyperintensities and FLAIR vascular hyperintensities–DWI mismatch was assessed on the 7 cortical ASPECTS regions, ranging from 0 (absence) to 7 (extensive), and associations with good collaterals were compared using receiver operating characteristic curves.
RESULTS: Of the 209 included patients, 133 (64%) and 76 (36%) had good and poor collaterals, respectively. All–FLAIR vascular hyperintensity extent was similar between collateral groups (P = .76). Conversely, FLAIR vascular hyperintensities–DWI mismatch extent was significantly higher in patients with good compared with poor collaterals (P < .001). The area under the curve was 0.80 (95% CI, 0.74–0.87) for FLAIR vascular hyperintensities–DWI mismatch and 0.52 (95% CI, 0.44–0.60) for all–FLAIR vascular hyperintensities (P < .001 for the comparison), to predict good collaterals. Variables independently associated with good collaterals were smaller DWI lesion volume (P < .001) and larger FLAIR vascular hyperintensities–DWI mismatch (P = .02).
CONCLUSIONS: In acute ischemic stroke with large-vessel occlusion, the extent of FLAIR vascular hyperintensities does not reliably reflect collateral status unless one accounts for DWI.
ABBREVIATIONS:
- AIS
- acute ischemic stroke
- ASITN/SIR
- American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology
- FVH
- FLAIR vascular hyperintensities
- HIR
- hypoperfusion intensity ratio
- IQR
- interquartile range
- IVT
- IV thrombolysis
- LVO
- large-vessel occlusion
- MT
- mechanical thrombectomy
- ROC
- receiver operating characteristic
- Tmax
- time-to-maximum
Neuroimaging is critical for the diagnosis and triage for treatment of patients with acute ischemic stroke (AIS) due to large-vessel occlusion (LVO). Collateral status before treatment is an important determinant of tissue fate and response to treatment.1,2 Incorporation of collateral flow status into clinical decision-making may help determine eligibility for mechanical thrombectomy (MT),3 particularly in the delayed time window.4
Collateral grading on DSA is considered the criterion standard. It is based on both the extent and delay of retrograde perfusion, as per the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) score.5,6 This score has been adapted to collateral flow maps derived from PWI source data. These collateral flow maps have been validated against DSA, and provide greater parenchymal detail.2,7
Besides this advanced MR imaging technique for direct collateral flow imaging,2,7 routine MR images without contrast injection potentially provide valuable information regarding collateral status. On the FLAIR sequence, intracranial arteries normally appear dark due to the loss of signal intensity from the movement of blood. In case of an arterial occlusion, intracranial arteries become brighter, presumably because of the slow retrograde flow through leptomeningeal channels.8 These FLAIR vascular hyperintensities (FVH) are defined as focal, tubular, or serpentine hyperintensities in the subarachnoid space relative to CSF,9 corresponding to a typical arterial course. Although FVH have been proposed as a surrogate marker of collateral status,9 the relation between FVH and collateral status remains incompletely understood.10,11 Actually, FVH may not only represent retrograde collateral flow but also reflect slow anterograde flow or even stationary blood.
Collateral grading based on FVH commonly considers the extent of all FVH (all–FVH) in the affected territory; however, this lacks information regarding brain parenchyma, in contrast to both the ASITN/SIR DSA criterion standard and the PWI-derived collateral flow map–based grading scores. Critically, in the latter scores, parenchymal defect is a key item for distinguishing poor from good collaterals. Thus, the extent of all–FVH without consideration of the brain parenchyma may imperfectly reflect collateral adequacy. In contrast, assessing the presence of FVH beyond the DWI lesion, the so-called “FVH-DWI mismatch,” considers only the FVH associated with the at-risk but not yet irreversibly damaged tissue.12 Accordingly, the FVH-DWI mismatch has been previously used as an alternative to the PWI-DWI mismatch to assess the at-risk tissue12,13 and identify patients most likely to benefit from intravenous thrombolysis (IVT)14 or MT.15,16
On the basis of the above rationale, we hypothesized that the FVH-DWI mismatch is a better surrogate of collateral status than considering all–FVH. To test this hypothesis, we compared the value of the FVH-DWI mismatch versus all–FVH for collateral assessment using PWI-derived collateral flow maps as references.
MATERIALS AND METHODS
Study Design and Inclusion Criteria
All patients in the present study were included in a previous study that reported that good collaterals independently predict post-IVT recanalization before MT.1 In accordance with French legislation, each patient was informed of his or her participation in the latter study and was offered the possibility to withdraw. Because the study implied retrospective analysis of anonymized data collected as part of routine care, formal approval by an ethics committee was not required. This manuscript was prepared according to the Standards for Reporting Diagnostic Accuracy (STARD) statement.17 Data from 6 French stroke centers that perform PWI as part of routine admission imaging (Sainte-Anne [Paris], Hospices Civils [Lyon], Orléans, Tours, Montpellier, and Nancy University hospitals), extracted from a large French multicenter registry (PREDICT-RECANAL) of consecutive patients with LVO stroke referred for MT after IVT,18 were reviewed. Of note, the PWI data were not a basis for decision-making routinely, except in borderline cases.
The following inclusion criteria were used for this study: 1) acute stroke with LVO of the anterior circulation treated with IVT and referred for MT between May 2015 (when MT became routine care in these centers) and March 2017; and 2) pre-IVT MR imaging including DWI, FLAIR, T2*, MRA, and dynamic susceptibility contrast PWI to compute collateral flow maps (see below).
Data Acquisition
The following variables were extracted from the registries: age, sex, vascular risk factors, NIHSS score on admission, time between symptom onset and the start of MR imaging (onset-to-MR imaging time), and time between symptom onset and the start of IVT (onset-to-IVT time).
In line with French recommendations,19 MR imaging is implemented as first-line imaging in candidates for reperfusion therapy in all centers of the present study. Per inclusion criteria, the stroke MR imaging protocol included DWI, T2*, TOF-MRA, and dynamic susceptibility contrast PWI. FLAIR sequences were those routinely used in each center and were not standardized. The list of the main parameters of FLAIR sequences is presented in the Online Supplemental Data (all 2D FLAIR sequences).
A stroke neurologist (P.S. with 7 years of experience in stroke MR imaging) reviewed the pre-IVT imaging of all included patients. We collected the following variables: 1) occlusion site, according to 3 categories (intracranial ICA T or L occlusion and M1 and M2 segments of the MCA); 2) DWI lesion volume, semiautomatically segmented using Olea Sphere (Olea Medical) after applying a threshold of 620 × 10−6 mm2/s on ADC maps,20 with manual correction whenever necessary; and 3) hypoperfusion volumes, with time-to-maximum (Tmax) >4-, >6- and >10-second volumes, automatically segmented from PWI using Olea Sphere, with manual correction whenever necessary.21 Mismatch ratio was considered as a continuous variable and defined as Tmax > 6-second volume/DWI volume.22 It was further dichotomized using a 1.8 cutoff (PWI-DWI mismatch profile: Tmax > 6-second volume >1.8 × DWI volume).23 The hypoperfusion intensity ratio (HIR) was defined as Tmax > 10-second/Tmax > 6-second volume. The HIR reflects the proportion of severely hypoperfused tissue and is another surrogate for collaterals.24
PWI Collateral Flow Map Generation and Grading
The PWI acquisition parameters used in each participating center have been previously detailed.1 The collateral grading method used here is similar to that described in Kim et al.2 Briefly, this method uses the PWI raw data set and subtracts the baseline prebolus image from each frame of the raw perfusion data to automatically generate 3 sets of maps covering the MCA territory (early phase map, mid phase map, and late phase map, corresponding to the arterial, capillary, and venous phases of angiography, respectively) from which collaterals are visually graded from 1 to 4 on the basis of the ASITN/SIR angiographic classification,5,6 relying on the extension and the delay of retrograde perfusion: grade 1 (no or slow collaterals to the periphery of the ischemic site with persistence of some of the defect), grade 2 (rapid collaterals to the periphery of the ischemic site with persistence of some of the defect), grade 3 (collaterals with slow but complete angiographic blood flow of the ischemic bed by the late venous phase), and grade 4 (complete and rapid collateral blood flow to the vascular bed in the entire ischemic territory by retrograde perfusion).1,2,7 This technique has previously been validated against DSA2 and was used here as reference for collateral assessment. Of note, the reproducibility of collateral grading on PWI collateral flow maps was previously assessed in a random subset (n = 100); the weighted κ value for interrater agreement was 0.85 (95% CI, 0.76–0.93).1
Extent of FVH-DWI Mismatch and of All–FVH
FVH were defined as focal, tubular, or serpentine hyperintensities in the subarachnoid space relative to CSF,9 corresponding to a typical arterial course. On the basis of the 7 cortical ASPECTS regions,25 DWI and FLAIR sequences were rated for the presence or absence of DWI lesions and FVH by 1 radiologist (A.L.B. with 5 years of experience), blinded to the collateral grade. This rating allowed us to compute FVHASPECTS ranging from 0 (no FVH) to 7 (FVH abutting all cortical ASPECTS regions)26,27 and FVH-DWI mismatchASPECTS ranging from 0 (no FVH-DWI mismatch) to 7 (FVH-DWI mismatch in all cortical ASPECTS areas).12 To assess reproducibility, an experienced neuroradiologist (L.L. with 8 years of experience) independently reviewed a random subset (n = 100) of the sample. Figures 1 and 2 illustrate good and poor collaterals, respectively.
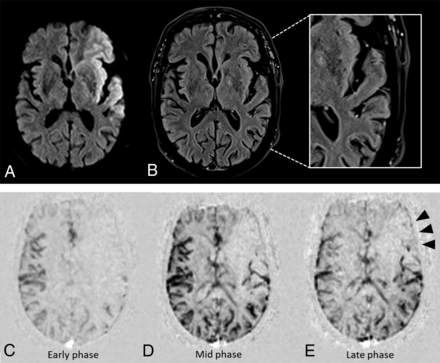
Illustrative case of good collaterals. MR imaging of a patient around 60 years of age obtained 108 minutes after stroke onset. Hyperintense lesions in the left MCA territory on admission DWI (A) and FVH on admission FLAIR (B), with FVHASPECTS of 5 and FVH-DWI mismatchASPECTS of 4. Grade 4 collaterals on early phase (C), mid phase (D), and late phase (E) of collateral flow maps (subtracted dynamic susceptibility contrast perfusion imaging). Note complete and rapid (from mid phase) collateral blood flow to the vascular bed in the entire ischemic territory by retrograde perfusion.
Illustrative case of poor collaterals. MR imaging of a patient around 70 years of age obtained 83 minutes after stroke onset. Hyperintense lesions in the left MCA territory on admission DWI (A) and FVH on admission FLAIR (B), with FVHASPECTS of 6 and FVH-DWI mismatchASPECTS of 1. Grade 2 collaterals on early phase (C), mid phase (D), and late phase (E) of collateral flow maps (subtracted dynamic susceptibility contrast perfusion imaging). Note rapid collaterals to the periphery of ischemic site with persistence of some of the defect (arrowheads).
Statistical Analysis
Categoric variables were described as number (percentage) and were compared using the χ2 or Fisher exact test. Continuous variables were described as means or median (interquartile range [IQR]) and compared using the Student t test or Mann-Whitney U test, as appropriate. The intraclass correlation coefficient was used to assess interobserver agreement for FVHASPECTS and FVH-DWI mismatchASPECTS. Correlations between collateral grades (1–4) and prespecified variables of interest (NIHSS, DWI lesion volume, PWI-DWI mismatch profile, and HIR) were assessed using the Spearman (ρ) coefficient. Associations between the dichotomized collateral status and FVH-DWI mismatchASPECTS or FVHASPECTS were assessed in univariable ordinal logistic regression and described as the common OR and its 95% CI, after verifying the assumption of proportional odds. Baseline variables at a level of P < .20 were candidates for inclusion in a multivariable binary logistic regression model, with good collaterals as the dependent variable. Variable selection was performed backwards using a stepwise approach, whereby candidate variables entered the model at P < .20 and were retained only if they remained associated at P < .05 with the dependent variable. Covariates were assessed for collinearity and interaction effects. To determine the predictive ability of FVH-DWI mismatch and all–FVH to assess collateral status, we calculated the area under the receiver operating characteristic (ROC) curve (ie, c-statistics). Statistical analyses were performed using SAS 9.4 (SAS Institute) and SPSS 19.0 (IBM). Two-tailed P < .05 was considered statistically significant.
RESULTS
Patient Characteristics, Collateral Grade, and Other Variables
Of the 224 patients reported in the princeps study,1 15 were excluded because MR imaging was not available (n = 4), lacked the FLAIR sequence (n = 8), or was affected by severe movement artifacts (n = 3), leaving 209 patients for the final analysis. Excluded patients were similar to included patients in terms of age (P = .21), sex (P = .46), NIHSS score (P = .40), occlusion site (P = .14), and collateral grade (P = .41). The baseline characteristics of included patients are presented in the Table. Grade 1 (poor) collaterals were present in 5 (2%); grade 2, in 71 (34%); grade 3, in 108 (52%); and grade 4 (excellent), in 25 (12%) patients. Given the small number of patients with grade 1 and 4 collaterals, grades 1–2 (poor collaterals) and grades 3–4 (good collaterals) were merged for subsequent analyses. A higher collateral grade was associated, as expected, with lower admission NIHSS scores (ρ = −0.29, P < .001), smaller DWI lesions (ρ = −0.61, P < .001), more frequent PWI-DWI mismatch profiles (96% versus 81%, P < .001), and lower HIR (ρ = −0.57, P < .001).
Baseline characteristics of the population and univariate relationships with collateral gradea
Extent of FVH-DWI Mismatch and of All–FVH
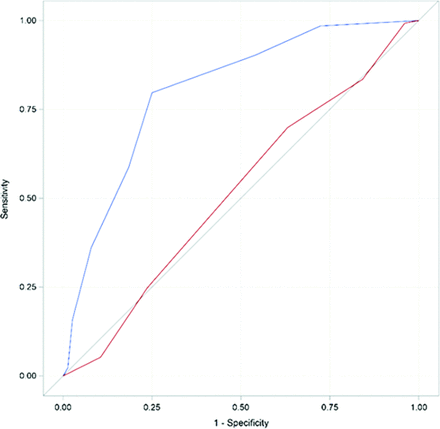
Interobserver agreement for FVHASPECTS and FVH-DWI mismatchASPECTS was good, with intraclass correlation coefficients of 0.83 (95% CI, 0.75–0.89) for FVHASPECTS, in line with previous studies,26,27 and 0.89 (95% CI, 0.84–0.92) for FVH-DWI mismatchASPECTS. FVH-DWI mismatchASPECTS was significantly larger in patients with good compared with poor collaterals (median: 4 [IQR, 3–5] versus 2 [IQR, 0–2.75], P < .001). Conversely, FVHASPECTS was similar between the 2 groups (median: 5 [IQR, 4–5.5] in the good collaterals group versus 5 [IQR, 4–5] in the poor collaterals group, P = .76, Table). The common OR for good collaterals was 1.75 (95% CI, 1.48–2.06, P < .001) for the FVH-DWI mismatch and 1.00 (95% CI, 0.80–1.25, P = .98) for all–FVH. The area under the ROC curve to predict good collaterals was 0.80 (95% CI, 0.74-0.87) for FVH-DWI mismatch and 0.52 (95% CI, 0.44-0.60) for all–FVH (P < .001 for the comparison) (Fig 3). The cut-point of FVH-DWI mismatchASPECTS, maximizing the sum of sensitivity and specificity, was ≥3. By means of this cut-point, FVH-DWI mismatchASPECTS identified good collaterals with a sensitivity of 80% (95% CI, 72%–86%), specificity of 75% (95% CI, 64%–84%), a positive predictive value of 85% (95% CI, 77%–90%), and a negative predictive value of 68% (95% CI, 57%–77%). We verified post hoc that the results were similar by using the HIR instead of the PWI-derived collateral flow maps as an alternative surrogate for collaterals: HIR was dichotomized on the basis of its median value (0.43) into low (ie, good collaterals) versus high (ie, poor collaterals) subgroups. The area under the ROC curves to predict low HIR was 0.75 (95% CI, 0.68–0.81) for FVH-DWI mismatch and 0.53 (95% CI, 0.45–0.61) for all–FVH (P < .001 for the comparison). We also studied post hoc the correlations between FVHASPECTS/FVH-DWI mis matchASPECTS and perfusion deficits using Spearman (ρ) coefficients (Online Supplemental Data). The extent of all–FVH was associated with larger perfusion abnormalities (irrespective of the severity of the hypoperfusion). In contrast, the extent of the FVH-DWI mismatch was not correlated with Tmax > 4-second and Tmax > 6-second volumes but was negatively correlated with Tmax > 10-second volume and HIR, consistent with FVH-DWI mismatch reflecting good collaterals.
ROC curves of FVH-DWI mismatchASPECTS (blue) and FVHASPECTS (red) to predict good (grades 3–4) collaterals. The area under the ROC curve was higher for FVH-DWI mismatchASPECTS (0.80; 95% CI, 0.74–0.87) than for FVHASPECTS (0.52; 95% CI, 0.44–0.60), P < .001 for comparison.
Multivariable Analysis
By means of good collaterals as the dependent variable, the multivariable model included 205 patients (132 with good collaterals and 73 without, after exclusion of 4 patients without PWI volumes). Variables included in the multivariable model were NIHSS score, DWI volume, Tmax > 6-second volume, and FVH-DWI mismatchASPECTS. Because of the collinearity among all PWI-derived metrics, only the Tmax > 6-second volume was entered in the model. Variables that remained independently associated with good collaterals were DWI volume (adjusted OR, 0.91; 95% CI, 0.87–0.94; P < .001) and FVH-DWI mismatchASPECTS (adjusted OR, 1.33; 95% CI, 1.04–1.70; P = .02); ie, patients with good collaterals had smaller DWI lesion volume and larger FVH-DWI mismatch.
DISCUSSION
In this large, multicentric population of patients with AIS referred for MT, we found, in line with our hypothesis, that the FVH-DWI mismatch is a reliable surrogate of collateral status and a better one than all–FVH. These findings emphasize the importance of taking into account the DWI lesion whenever FVH are used as a surrogate of collateral status.
Collaterals play a major role in stroke pathophysiology.28 These alternative vascular channels maintain perfusion to the ischemic tissue distal to the arterial occlusion, to a degree that depends on their strength. Accordingly, collateral status is a key determinant of the speed of core growth before recanalization2 and may be used for decision-making as a major treatment effect modifier.4 Although DSA remains the reference to assess collateral status, it is invasive; furthermore, exploration of all 4 arterial axes during MT is not systematically performed to save time, thereby hampering a comprehensive analysis of collaterals. Moreover, interobserver agreement of ASITN/SIR collateral grading on DSA has been reported by some to be poor.29 Finally, DSA provides information on collaterals once the patient is in the catheterization lab, which has a limited impact on the endovascular treatment workflow.
One strength of our study is the use of PWI for collateral assessment, as also reported by others.1,2,7,30 Contrary to DSA, grading on PWI-derived collateral flow maps has an almost perfect interobserver agreement.1,30 Moreover, PWI is performed within minutes of FLAIR, thereby providing information on collaterals almost simultaneous with FVH visualization. On the downside, PWI is not consistently part of acute stroke MR imaging because it requires additional scanning time, contrast agent administration and dedicated software. Thus, a straightforward pretreatment surrogate of collateral status would be useful in the clinical setting. This surrogate should be easily extractable from standard sequences whenever MR imaging is used for patient triage and should be directly assessable before patient transfer to the catheterization lab. FVH are such a promising candidate.
Our findings are consistent with accumulating evidence that FVH are a marker of adequate collaterality10 and, furthermore, that FVH beyond and those facing the DWI lesion have a different clinical significance,31 as recently highlighted in a study-level meta-analysis including 36 cohort studies involving >3500 patients.32 Studies that separately analyzed the FVH beyond versus those facing the DWI lesion found that the FVH pattern, not their sole extent or number, could serve as an imaging-selection criterion for endovascular therapy.31,33 Our study is the first to directly compare the 2 main methods of FVH assessment for collateral assessment in the same population. To this end, we used the same scoring approach based on ASPECTS regions for both all–FVH and FVH-DWI mismatch and found that good collaterals were associated solely with FVH-DWI mismatch extent. This finding relied on PWI-derived collateral flow maps to estimate collateral status and was confirmed post hoc using HIR as alternative collateral surrogate. Our finding is consistent with those in previous studies reporting that patients with an FVH-DWI mismatch have higher ASITN/SIR scores, ie, better collaterals than those without an FVH-DWI mismatch.34,35 These studies, however, considered the FVH-DWI mismatch as a binary variable compared with an ordinal one as used here. Samples used were small and overlapping, and interobserver reproducibility of collateral assessment on DSA was lacking.
Here we found no significant association between all–FVH extent and collateral grade, in contrast to some previous studies using DSA as a reference.36,37 However, the DSA-based studies used small samples, did not report interobserver reproducibility of DSA-based collateral assessment, and found no association between extensive all–FVH and smaller infarct size, which seems inconsistent. Using HIR rather than DSA before the MT era, a study found that all–FVH extent was associated with good collaterals.26 However, the FVH-DWI mismatch was not assessed in this monocenter study. Our multicenter study suggests that in patients referred for MT, the latter is a much better surrogate of collaterals than all–FVH. A score based on FVH-only might be more sensitive to data heterogeneity than a score based on the FVH-DWI mismatch, given that DWI lesion visibility is more straightforward than FVH, which are influenced by FLAIR parameters.38
Overall, the FVH facing and those beyond the DWI lesion may not share the same pathophysiology. In support, all–FVH had no prognostic value in 2 previous studies;31,39 furthermore, the presence of FVH within the DWI lesion was associated with subsequent hemorrhagic transformation.31 Considering this previous work together with our present findings, it would appear that all–FVH, though still widely used in recent prognostic studies,27,40 do not reliably reflect the collateral status in MT candidates and may, instead, represent variable degrees of perfusion abnormalities.
Our study has limitations. First, the multicenter registry used here was not designed for our analysis. Second, because our study included patients who underwent PWI, a selection bias cannot be excluded. However, patients excluded because of absent or poor-quality PWI did not differ from included patients in terms of age, NIHSS scores, and occlusion site.1 Third, MR images were mainly obtained within 4 hours of stroke onset, and results might differ at later time points when information on collaterals might be more useful for treatment decisions.10,11 Fourth, some patients were excluded because of FLAIR artifacts; however, this exclusion concerned <2% of the total sample. Fifth, although ratings of FVHASPECTS and FVH-DWI mismatchASPECTS remain subjective, interobserver agreement was good. Last, there were differences in FLAIR and DWI sequences among centers, which could have influenced the assessment of the FVH-DWI mismatch. Nevertheless, the prevalence of the FVH-DWI mismatch was similar across magnetic field strengths and manufacturers in a previous cohort,15 suggesting that it may be applied regardless of the MR imaging unit.
CONCLUSIONS
On the basis of a large multicenter population of patients with AIS and LVO who underwent MR imaging before MT, we show that the extent of the FVH-DWI mismatch is a good surrogate for collateral status and better than all–FVH. These findings support our hypothesis that the DWI lesion should be considered whenever FVH are used as surrogates for collaterals in this setting.
Acknowledgments
We thank Fatemehsadat Arzanforoosh for the collection of the technical parameters for FLAIR sequences in the participating centers.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received July 29, 2022.
- Accepted after revision October 27, 2022.
- © 2023 by American Journal of Neuroradiology