Abstract
BACKGROUND AND PURPOSE: Flat panel detector CT imaging allows simultaneous acquisition of multiphase flat panel CTA and flat panel CTP imaging directly in the angio suite. We compared collateral assessment derived from multiphase flat panel CTA and flat panel CTP with collateral assessment derived from DSA as the gold-standard.
MATERIALS AND METHODS: We performed a retrospective analysis of patients with occlusion of the first or second segment of the MCA who underwent pre-interventional flat panel detector CT. The hypoperfusion intensity ratio as a correlate of collateral status was calculated from flat panel CTP (time-to-maximum > 10 seconds volume/time-to-maximum > 6 seconds volume). Intraclass correlation coefficients were calculated for interrater reliability for the Calgary/Menon score for multiphase flat panel CTA and for the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) score for DSA collateral scores. Correlations of the hypoperfusion intensity ratio, multiphase flat panel CTA score, and the ASITN/SIR score were calculated using the Spearman correlation.
RESULTS: From November 2019 to February 2020, thirty patients were included. Moderate interrater reliability was achieved for the ASITN/SIR DSA score (0.68; 95% CI, 0.50–0.82) as well as for the Calgary/Menon multiphase flat panel CTA score (0.53; 95% CI, 0.29–0.72). We found a strong correlation between the ASITN/SIR DSA and Calgary/Menon multiphase flat panel CTA score (ρ = 0.54, P = .002) and between the hypoperfusion intensity ratio and the Calgary/Menon multiphase flat panel CTA score (ρ = −0.57, P < .001). The correlation was moderate between the hypoperfusion intensity ratio and the ASITN/SIR DSA score (ρ = –0.49, P = .006). The infarct core volume correlated strongly with the Calgary/Menon multiphase flat panel CTA score (ρ = –0.66, P < .001) and the hypoperfusion intensity ratio (ρ = 0.76, P < .001) and correlated moderately with the ASITN/SIR DSA score (ρ = –0.46, P = .01).
CONCLUSIONS: The Calgary/Menon multiphase flat panel CTA score and the hypoperfusion intensity ratio correlated with each other and with the ASITN/SIR DSA score as the gold-standard. In our cohort, the collateral scoring derived from flat panel detector CT was clinically reliable.
ABBREVIATIONS:
- ASITN/SIR
- American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology
- FDCT
- flat panel detector CT
- FDCTP
- flat panel detector CTP
- HIR
- hypoperfusion intensity ratio
- ICC
- intraclass correlation coefficient
- mCTA
- multiphase CTA
- mFDCTA
- multiphase flat panel detector CTA
- rCBF
- relative CBF
- Tmax
- time to maximum
In acute ischemic stroke, sufficient and effective collaterals are important, correlate with favorable outcome, and may be used for patient selection.1-3 The gold-standard American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) score for DSA4 is the most commonly used scale for visual collateral scoring.5 Two alternative collateral scores are the Calgary/Menon score for multiphase CTA (based on visual inspection)6 and the hypoperfusion intensity ratio (HIR) as a quantitative and thus objective estimation of the collateral supply. The HIR can be derived from perfusion imaging and is defined as the ratio of the volume of tissue with time to maximum (Tmax) > 10 seconds to the volume of tissue with Tmax >6 seconds.7
The recently introduced whole-brain multiphase flat panel detector CT (FDCT) imaging allows acquisition of time-resolved whole-brain perfusion imaging directly in the angio suite by 10 rotational sweeps. On the basis of flat panel detector CTP (FDCTP) source images, multiphase flat panel detector CTA (mFDCTA) and perfusion maps can be reconstructed.8-10 This technology enables direct transport of patients to the angio suite (“direct-to-the angio” approach), which, in turn, can improve in-hospital workflows, reduce the time to treatment, and may ultimately increase the chances of good clinical outcome.11-16
While collateral scores are well-validated in conventional multiphase CTA (mCTA)/CTP, data on the evaluation of the collateral status derived from FDCTP imaging is scarce. FDCTP offers the unique possibility of validating and comparing cross-sectional collateral surrogates with the conventional DSA gold-standard, acquired at almost the same time.
The purpose of this study was to evaluate collateral imaging derived from FDCT as a cornerstone in its possible future use as a first-line stroke imaging technique (direct-to-the angio approach). We assessed interrater reliability and correlation of collateral scores derived from FDCTP (Calgary/Menon mFDCTA score, HIR) and from DSA (ASITN/SIR DSA score). Furthermore, we assessed the correlation of collateral scores with the size of infarct cores.
MATERIALS AND METHODS
The study protocol was approved by the Kantonale Ethikkommission Bern (reference ID 2018-02023). Informed consent was waived owing to the retrospective nature of the study. The data that support the findings of this study are available from the corresponding author on reasonable request.
Population
We included patients from November 2019 to February 2021 with M1 and M2 occlusions who underwent mechanical thrombectomy at our institution and pre-interventional FDCTP. Exclusion criteria were low-quality (qualitative and quantitative features not interpretable, eg, due to delayed contrast bolus arrival) FDCTP scans. Clinical data were extracted from the institutional stroke database or from the Clinical Information System.
Imaging Protocol
After neurologic examination on arrival at the emergency department, patients underwent either CT imaging (noncontrast CT, CTA, CTP) or MR imaging (DWI, FLAIR, SWI, MRA, MR Perfusion) and were subsequently transferred to the angio suite if the eligibility criteria for mechanical thrombectomy were fulfilled. The decision as to whether to perform pre-interventional FDCT imaging was at the discretion of the treating physician based on clinical reasoning. FDCT imaging provided additional information about the penumbra and infarct core volumes and collateral status, which allowed adjusting the treatment strategy accordingly.
FDCT imaging was performed with the patient under general anesthesia. Whole-brain imaging data were acquired using a biplane flat panel detector angiographic system (Artis icono; Siemens) by 10 rotational sweeps (each during 5 seconds) of the angiographic C-arm system around the patient. The first 2 sweeps served as mask runs, and the following 8 rotations recorded in-/outflow of contrast agent (60 mL, Iopamiro 400; Bracco), which was started at the same time as the mask run via an 18G left or right cubital venous line. Finally, a 40-mL saline flush was administered (power injector injection rate, 5 mL/s).
Postprocessing of the raw perfusion imaging was conducted using a dedicated software (Rapid ANGIO; iSchemaView), and mFDCTA as well as several perfusion maps were reconstructed (relative CBF [rCBF], relative CBV [rCBV], MTT, and Tmax). On the basis of these maps, volumes of tissue with Tmax > 6 seconds, Tmax > 10 seconds, and rCBF < 45% were calculated. Rapid ANGIO is the first and currently only clinically available postprocessing software for multiphase FDCTP acquisitions based on the Rapid software.11
Thick-section axial MIPs at 4-mm thickness and 1-mm intervals of the mFDCTA were reconstructed. Because 10 phases were available for each mFDCTA, 3 phases were defined to mimic a conventional mCTA (to apply the Calgary/Menon mCTA score, see the “Imaging Analysis” section). The arterial phase was defined as the phase in which the arterial vessels were best depicted due to signal increase caused by inflow of contrast agent. The second phase was defined as 6 seconds after the arterial phase, and the third phase, as 12 seconds after the arterial phase.
Infarct core was defined as tissue with rCBF of <45% because phantom data showed that FDCTP is less sensitive than conventional CTP for detecting CBF values of <30 mL/100 g/min. Furthermore, a pilot study in patients with acute stroke demonstrated a strong correlation with conventional CTP (Pearson = 0.91, Spearman = 0.87) with an intraclass correlation coefficient (ICC) of 0.89 (95% CI, 0.67–0.96) using the <45% threshold for FDCTP.17 The HIR was defined as the ratio of the Tmax > 10-second volume divided by the Tmax > 6-second volume.18
Imaging Analysis
Two experienced interventional neuroradiologists (E.I.P., S.P.P.) and 1 neuroradiology resident (C.C.K.), who were blinded to other clinical data performed ratings of the collaterals on DSA (biplane flat panel detector angiographic system) and mFDCTA. After the initial rating, a consensus rating was determined for all cases with discrepant ratings in a joint session. Images were not anonymized, but initial and consensus ratings were performed separately for DSA and mFDCTA with 1 month of washout between them.
For the DSA, we used the 5-point ASITN/SIR collateral flow grading system:4 grade 0, no collaterals visible to the ischemic region; grade 1, slow collaterals to the periphery with persisting defect; grade 2, rapid collaterals to the periphery with persisting defect; grade 3, slow-but-complete collateral flow to the ischemic territory; and grade 4, rapid and complete collateral flow to the ischemic territory.
For the mFDCTA, we used the 6-point Calgary/Menon score:6 0, no filling in any phase within the ischemic territory; 1, just a few vessels visible in any phase in the affected territory; 2, delay of 2 phases in the filling of peripheral vessels and decreased extent or a 1-phase delay and some ischemic regions without vessels; 3, delay of 2 phases in the filling of peripheral vessels or a 1-phase delay and reduced number of vessels in the ischemic territory; 4, delay of 1 phase in filling of peripheral vessels, with prominence and extent the same; and 5, no delay and normal or increased prominence of pial vessels.
Statistical Analysis
Normal distribution was tested using graphic distribution and the Shapiro-Wilk test. Data are displayed as number (percentage) and median (interquartile range) if not otherwise specified. No data of the examined variables were missing.
The ICC was used to calculate interrater reliability because it is fit to handle ordinal data.19 ICC estimates and their 95% confidence intervals were calculated on the basis of a single rating, absolute agreement, and the 2-way random effects model. Interpretation was as follows: <0.5, poor; 0.5–0.75, moderate; 0.75–0.9, good; and >0.9, excellent reliability.19 As proposed by Koo et al,19 categories of agreement were determined on the basis of 95% confidence intervals, and if confidence intervals included 2 categories, agreement was described as transition (eg, moderate-to-good).
For correlation analysis, the consensus ratings for ASITN/SIR DSA scores and Calgary/Menon mFDCTA scores were used. Correlations were calculated using the Spearman correlation because the Spearman correlation can handle ordinal and non-normally distributed data. Spearman correlation coefficients were interpreted as follows: ρ < 0.3, weak relationship; 0.3 ≤ ρ ≤ 0.5, moderate relationship; ρ > 0.5, strong relationship.20
In boxplot analyses, overall differences were compared using the Kruskal-Wallis test.
All statistical analyses were conducted using R (Version 4.0.2; http://www.r-project.org/).21 A 2-tailed P value < .05 was considered statistically significant.
RESULTS
Population
Of 36 patients, we excluded 6 patients due to bad FDCTP quality caused by delayed bolus arrival (n = 5) or a dispersed bolus (n = 1), leaving a final population of 30 patients for the final analysis (Online Supplemental Data). The median age was 76.5 years, 40% were women, and 21 patients presented with M1 occlusions. Baseline characteristics and distribution of patients according to the ASITN/SIR DSA score and the Calgary/Menon mFDCTA score are shown in the Online Supplemental Data.
Interrater Reliability
Interrater reliability was moderate-to-good for the ASITN/SIR DSA score (ICC estimate, 0.68; 95% CI, 0.50–0.82) and poor-to-moderate for the Calgary/Menon mFDCTA score (ICC estimate, 0.53; 95% CI, 0.29–0.72). When considering only M1 occlusions, interrater reliability improved for the ASITN/SIR DSA score (ICC estimate, 0.75; 95% CI, 0.56–0.88) and for the Calgary/Menon mFDCTA score (ICC estimate, 0.66; 95% CI, 0.43–0.83).
Correlation of Collateral Scores
The Online Supplemental Data show the distribution of ASITN/SIR DSA versus Calgary/Menon mFDCTA consensus scores for each case.
We found strong correlations between ASITN/SIR DSA score and Calgary/Menon mFDCTA score (ρ = 0.54, P = .002) and between the HIR and the Calgary/Menon mFDCTA score (ρ = −0.57, P <.001), and moderate correlation between the HIR and ASITN/SIR DSA score (ρ = −0.49, P = .006). Again, when we considered only M1 occlusions, correlations among all scores were strong: between the ASITN/SIR DSA score and Calgary/Menon mFDCTA score (ρ = 0.65, P = .001), between the HIR and Calgary/Menon mFDCTA score (ρ = −0.56, P = .008), and between HIR and ASITN/SIR DSA score (ρ = −0.62, P = .003).
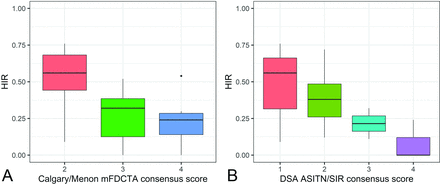
Comparing the HIR for distinct Calgary/Menon mFDCTA degrees revealed a trend for lower HIR values in increasing Calgary/Menon mFDCTA scores (overall difference, P = .006; Fig 1A). The same trend was present for the different ASITN/SIR DSA scores (overall difference, P = .035; Fig 1B).
Boxplots showing HIR versus Calgary/Menon mFDCTA consensus scores (A) and HIR versus ASITN/SIR DSA consensus scores (B).
Illustrative cases are shown in Fig 2.
A–D, Patient with M1 occlusion (left) and good collaterals visualized (A) on pre-interventional mFDCTA (arterial phase, Calgary/Menon grade 4). Pre-interventional DSA (left: anterior-posterior view; right: lateral view) in the early arterial phase (B) and in a later venous phase (C) shows good leptomeningeal retrograde filling of the initial antegrade capillary blush deficit (target downstream territory, ASITN/SIR grade 4). D, Tmax perfusion maps on different levels that depict the low ratio of tissue with Tmax > 10 seconds (red) to Tmax > 6 seconds (green) (HIR = 0.2). E–H, Patient with M1 occlusion (left) and bad collaterals depicted (E) on pre-interventional mFDCTA (arterial phase, Calgary/Menon grade 2). Pre-interventional DSA (left: anterior-posterior view; right: lateral view) in the early arterial phase (F) and in a later venous phase (G) shows poor leptomeningeal retrograde filling of the initial antegrade capillary blush deficit (target downstream territory, ASITN/SIR grade 1). H, Tmax perfusion maps show the high ratio of tissue with Tmax > 10 seconds (red) to Tmax > 6 seconds (green) (HIR = 0.7).
Correlation of Collateral Scores and Infarct Core
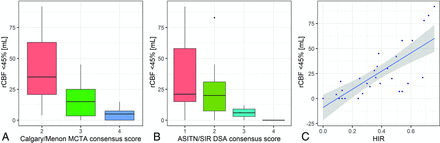
We also found a trend of decreasing infarct volumes with better collateral scores (Fig 3). This trend was present for the Calgary/Menon mFDCTA score (overall difference, P = .002; Fig 3A) as well as for the ASITN/SIR DSA score (overall difference, P = .03; Fig 3B). Correlation with rCBF of <45% was moderate for the ASITN/SIR (ρ = −0.46, P = .01), strong for the Calgary/Menon (ρ = −0.66, P <.001), and strong for the HIR (ρ = 0.76, P <.001; Fig 3C).
Boxplots showing rCBF < 45% versus Calgary/Menon mFDCTA consensus scores (A) and rCBF < 45% versus ASITN/SIR DSA consensus scores (B). C, Scatterplot with HIR versus rCBF < 45%.
DISCUSSION
This exploratory study has the following main findings: 1) Interrater reliability was moderate for the ASITN/SIR DSA score and the Calgary/Menon mFDCTA score, 2) collateral grading derived from FDCTP (mFDCTA, HIR) correlated with collateral grading derived from DSA as the gold-standard, and 3) collateral scores correlated with the size of infarct core.
Similar to previous studies, interrater reliability was only moderate for the ASITN/SIR DSA collateral score and the Calgary/Menon score for mFDCTA. Interrater reliability for the Calgary/Menon score of conventionally acquired mCTA was also moderate in the study of Kauw et al22 but excellent in other studies.6,23 A reason for our lower interrater reliability could be the inclusion of M2 occlusions in our study because it was not the case in the study of Lyndon et al23 and was not clear in the study of Menon et al.6 Additionally, our interrater reliability improved in both modalities when only considering M1 occlusions. Interrater reliability for the ASITN/SIR DSA collateral score for 19 readers was only poor in another study (generalized κ = 0.16).24
In a study including 24 patients, Maier et al9 found a strong correlation between the Calgary/Menon mFDCTA collateral score and the ASITN/SIR DSA collateral score (Pearson correlation coefficient, 0.86). However, collaterals were not graded by consensus but by a single rater. For trichotomized (poor, intermediate, excellent) mCTA and DSA scores, a Spearman correlation coefficient of 0.827 was reported.25 One explanation for our lower correlation of the Calgary/Menon mFDCTA score and the ASITN/SIR DSA score could be that we included a considerably higher proportion of M2 occlusions (30% in our study versus 18.5%25 and 4.2%,9 respectively). Another reason could be some incomplete pre-interventional DSA runs in which the selected DSA of the nonoccluded cervical and intracranial vessels were not performed, and consequently, the examination of collaterals and cross-flow was limited. This point, on the other hand, highlights an important advantage of mFDCTA, in which imaging of collaterals can be performed immediately pre-intervention with a single injection of contrast agent instead of selective angiographies of different vessels with the potential to cause complications as for example dissections or emboli.
Guenego et al26 found a significant correlation of the HIR derived from conventional CTP maps and DSA collaterals (Pearson correlation coefficient, −0.327; P = .01). Our correlation was substantially better, likely because we calculated the HIR from FDCTP in the angio suite, which was performed only a few minutes before the pre-interventional DSA. The correlation of the HIR and mFDCTA reached similar correlation as HIR and mCTA derived from CT (Pearson correlation coefficient, −0.55; P < .001),23 highlighting again the clinical comparability of FDCT and CT. Imperfect interrater reliability is certainly a limitation of visual collateral assessment, and the particular strength of the HIR derived from FDCTP is that it provides an objective surrogate of collateral status that is also relatively easy to interpret.
The association of good collaterals and small infarct core is well-known.27 We could confirm these findings because the Calgary/Menon mFDCTA collateral score showed a strong correlation with the infarct core (rCBF <45%), similar to previous findings examining conventional CTP.28 Corroborating these results, we also found a strong correlation between the HIR and infarct core. Last, correlation of the ASITN/SIR DSA score and infarct core was moderate but nonetheless significant.
Our results show that measures of collateral status derived from FDCTP, namely mFDCTA and HIR, are clinically reliable and reached similar results compared with collateral scoring derived from CT in other studies. FDCTP collateral imaging provides some advantages over conventional CT/MR collateral imaging. First, it is acquired in the angio suite and, therefore, omits the delay between imaging and intervention caused by transfer of the patient. Second, mFDCTA makes selective DSA of nonoccluded vessels dispensable, potentially reducing the amount of contrast agent required and reducing the time to recanalization. Third, it allows the acquisition of 8 angiographic phases instead of 3 phases in the mCTA, providing more detailed information with better resolution in time of collateral flows.
Another important advantage of FDCTP is that it allows acquisition of nonenhanced brain CT, mFDCTA, and qualitative and quantitative perfusion maps simultaneously instead of the separately acquired sequences of CT/MR imaging.
Motion artifacts can have a severe negative impact on FDCTP imaging. Because it is our institutional guideline to perform all mechanical thrombectomies with the patient under general anesthesia, we were able to prevent the occurrence of motion artifacts and case exclusion was restricted to problems with the application of the contrast agent. Performing FDCTP without general anesthesia could compromise image quality and is a problem that remains to be resolved.
However, if technically adequate, pre-interventional FDCTP allows ruling out intracranial hemorrhage, determination of vessel occlusions, and, as we showed, assessing the collateral situation and estimating infarct core size. Our findings encourage the direct-to-the-angio approach, in which the patient is transported directly to the angio suite bypassing conventional CT or MR imaging. This approach can optimize in-hospital workflows, reduce time to treatment, and may ultimately improve clinical outcome.11-16 Furthermore, FDCTP imaging after incomplete recanalization could add significant value, for example, in deciding whether to extend thrombectomy or to stop.
Limitations
This study is limited by its retrospective and single-center study design. Furthermore, we did not include patients consecutively and present results of a relatively small and highly selected sample size. Due to our small sample size, we also did not cover all degrees of the ASITN/SIR DSA and Calgary/Menon mFDCTA collateral scores. However, other studies also reported only very few patients with either very high or very low collateral scores, representing a regular distribution with most patients with moderate-to-good collaterals. Last, we included only patients with large-vessel occlusions of the anterior circulation, and validation of FDCTP-derived collateral assessment in vessel occlusions of the posterior circulation is still needed. In summary, larger and prospective studies would be needed to overcome these limitations.
CONCLUSIONS
In our cohort, the Calgary/Menon mFDCTA collateral score and the HIR derived from FDCTP have good correlation with the ASITN/SIR DSA collateral score as gold-standard and with each other. Collateral scoring derived from FDCTP, especially the HIR as an objective measurement, is a promising tool to evaluate collateral status, but larger studies are needed to confirm our findings. In addition, our results support the implementation of the direct-to-the-angio approach, potentially reducing time to recanalization.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received June 17, 2022.
- Accepted after revision August 25, 2022.
- © 2022 by American Journal of Neuroradiology