Abstract
BACKGROUND AND PURPOSE: Established Doppler parameters for carotid stenosis assessment do not reflect North American Symptomatic Carotid Endarterectomy Trial (NASCET)-style methodology. We derived a Doppler parameter, termed sonographic NASCET index (SNI), and hypothesized that the SNI would provide greater angiographic correlation and better accuracy in predicting stenosis of 70% or greater than that of currently used peak systolic velocity (PSV) measurements.
METHODS: Inclusion criteria of angiographically proved carotid stenoses of 40–95% and measured proximal and distal internal carotid artery Doppler PSV values were established. Occlusions and near occlusions were specifically excluded. Doppler and angiographic data meeting the inclusion criteria from 32 carotid bifurcations were identified; actual angiographic stenoses ranged 40–89%. SNI values were calculated for each vessel. PSV and SNI were correlated with angiography by using linear regression analysis. Accuracies of SNI and PSV in predicting stenosis of 70% or greater were compared at two thresholds.
RESULTS: Correlation between SNI and angiography was superior to that between PSV and angiography (r2 = 0.64 vs 0.38). PSV and SNI values that corresponded to 70% angiographic stenosis were 345 cm/s and 45.5, respectively. Accuracy of PSV of 345 cm/s or greater in predicting stenosis of 70% or greater was 78%, compared with 88% for SNI of 45.5 or greater. The SNI value that corresponded to a PSV threshold of 250 cm/s was 33. Accuracy of PSV of 250 cm/s or greater in predicting stenosis of 70% or greater was 81%, compared with 88% for SNI of 33 or greater.
CONCLUSION: Correlation between SNI and angiography was greater than that between PSV and angiography. Accuracy of SNI in predicting stenosis of 70% or greater was also superior to that of PSV at two thresholds. These results suggest that SNI may be a better predictor of high-grade carotid stenosis than is PSV.
Vascular sonography is a safe, convenient, and relatively inexpensive means of evaluating atheromatous disease of the extracranial carotid arteries. Numerous studies have demonstrated the ability of sonography to help grade carotid stenosis, with accuracy rates approaching or exceeding 90% (1–15). Despite the emergence of new technologies such as CT angiography, duplex sonography remains a relatively accurate and noninvasive means of selecting surgically significant carotid stenoses. Sonography, alone or in combination with MR angiography, probably remains the most widely used initial method for preoperative evaluation at most medical centers worldwide (16–17). According to the Society of Radiologists in Ultrasound (SRU) consensus statement published in 2003, Doppler sonography is increasingly becoming the sole imaging technique used before surgery for the evaluation of carotid stenosis (18). In fact, the SRU panelists estimated that as many as 80% of patients in the United States undergo carotid endarterectomy after a sonographic examination as the only preoperative imaging study. Thus, it is extremely important that sonographic evaluation provide the most accurate possible results.
Elevation of the internal carotid artery (ICA) peak systolic velocity (PSV) has been shown to be the single most useful Doppler sonographic parameter for detecting hemodynamically significant carotid stenosis and for selecting patients for carotid endarterectomy (19–21). However, Doppler sonography has previously been shown to be unreliable in the quantification of stenosis severity as compared with the reference standard of arteriography, regardless of whether PSV alone or a ratio of ICA PSV to common carotid artery (CCA) PSV is considered (21, 22). However, the difference in benefit of carotid endarterectomy between the moderate stenosis category (50–69%), where only modest benefit is achieved, and the high-grade stenosis category (≥70%), where significant benefit is achieved, underscores the importance of accurate risk stratification by using sonography (23).
The diagnostic accuracy of PSV in relation to North American Symptomatic Carotid Endarterectomy Trial (NASCET) angiographic stenosis measurement is limited in part by a significant difference in methodology. Historically, practitioners of vascular sonography have attempted to assess the degree of carotid stenosis through the use of Doppler parameters that incorporate flow velocity measured at a single point along the proximal ICA, such as PSV or end-diastolic velocity (EDV). Alternatively, a simple ratio comparison has been used that incorporates data from the distal CCA: the ICA PSV–to–CCA PSV ratio (VICA/VCCA ratio). In either case, these parameters ignore flow velocity information from the distal “normal” ICA and, therefore, do not reflect the NASCET methodology of carotid stenosis quantification, which relies on a ratio of the diseased lumen to the more normal ICA lumen (24).
It has been suggested that inclusion of velocity data from a second point along the downstream normal ICA would be useful to improve the accuracy of Doppler sonography in the quantification of stenosis, akin to the NASCET method for measurement of angiographic stenosis (25). A simple ratio between the ICA systolic velocity at the carotid bulb and the distal ICA systolic velocity has been previously studied, and a slight improvement over PSV in predicting certain types of stenoses was found (26).
We have proposed a new Doppler parameter based on the NASCET-style methodology of stenosis quantification that is herein called the sonographic NASCET index (SNI). Our derivation of the SNI incorporates flow velocity measurements obtained from within the normal distal ICA through application of the mass balance principle of bulk flow in a mathematically rigorous fashion. We have compared the diagnostic accuracy of the SNI with PSV by means of a retrospective analysis of 32 carotid bifurcations, with use of conventional angiography as the reference standard. Our hypothesis was that the SNI would be both more sensitive and more accurate than PSV for the diagnosis of high-grade carotid stenoses, as the SNI has been specifically derived to reflect the NASCET methodology of stenosis measurement.
Methods
Material
A review of all carotid angiographic studies reported at our institution between October 1992 and July 1999 was performed. Vessels that were evaluated with both sonography and conventional arteriography were identified, with initial inclusion criteria of angiographically proven stenosis in the range of 40–95%, as well as measured proximal and distal ICA Doppler PSV values. The lower bound was chosen to exclude insignificant degrees of luminal narrowing. The upper bound was chosen to exclude occlusions and near occlusions with partial luminal collapse, because such vessels do not submit to accurate NASCET-style measurements and may have paradoxically low PSV values (18, 24). Also, sonographic evaluation of near occlusions is based primarily on gray-scale and color Doppler imaging, rather than velocity data, making such vessels unsuitable for inclusion in this study (18). From this subgroup, 32 ICAs with stenoses ranging between 40% and 89% as determined with arteriography by using the NASCET methodology were identified (32 ICAs in 27 patients). Vessels were excluded if an intervening carotid endarterectomy was performed. The average time interval between sonography and arteriography was 2 months. Original sonographic studies were reviewed on a picture archiving and communication system workstation to obtain flow velocity data that had been recorded at the time of the study. Only archived data that remained accessible to the investigators were available for inclusion in this study. Vessels wherein the reported PSV corresponded to the distal-most velocity observed within the ipsilateral ICA were excluded.
Angiography
Digital subtraction angiography was performed through a femoral artery approach, with selective injections in the CCAs. At least two orthogonal views of each carotid bifurcation were obtained. Delayed imaging and prolonged injections were performed for all patients. Technical considerations included an exposure rate of one image per second for 20 seconds or less and a manual injection volume of 20 mL or less of contrast material (Isovue 300 [iopamidol]; Bracco Diagnostics, Milan, Italy). In each case, the digital subtraction angiograms were reviewed in a blinded fashion by two experienced neuroradiologists (G.M.H., S.M.E.), and the final results were determined by consensus by averaging the two independent measurements for each vessel. Angiographic percentage stenosis determination was made in accordance with published NASCET guidelines (27).
Sonography
Carotid sonography was performed by experienced technologists in a single accredited laboratory at our institution, and the sonograms were interpreted by an experienced sonologist. Commercially available equipment that was state-of-the-art during the time period encompassed by this study was used for all examinations (Advanced Technology Laboratories, Bothell, WA; Acuson, Mountain View, CA). Five- or 7.5-MHz linear-array transducers were used, as dictated by patient body habitus. All images were obtained in accordance with an established laboratory protocol. All patients underwent gray-scale as well as color and spectral Doppler imaging. Angle adjustment was based on flow direction as depicted by color Doppler. Angle-adjusted spectral Doppler samples were obtained from predetermined sites within each CCA and ipsilateral ICA, including proximal, middle, and distal sites along the course of each vessel. The highest angle-adjusted velocities observed within each of the proximal, middle, and distal segments of the ICA were routinely recorded by the technologist, and the highest of these recorded velocities was routinely reported as the PSV by the interpreting radiologist. Doppler parameters routinely evaluated and reported for each carotid bifurcation included PSV, EDV, and VICA/VCCA ratio.
Definition and Derivation of the SNI
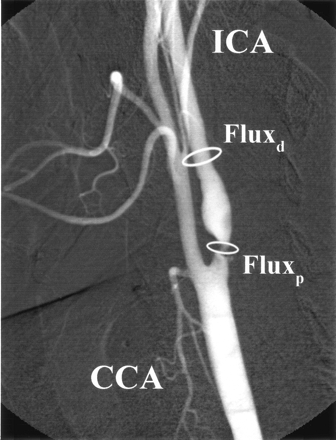
According to the principle of mass balance, the net flux at two different points along a single, nonbranching vessel must remain equal. With regard to the ICA, the flux proximally at point p (the point of maximal stenosis in the ICA) must equal the flux distally at point d (a more distal point along the normal ICA lumen). This is illustrated in Figure 1.
Left ICA angiogram (lateral projection) shows high-grade (≥70%) proximal ICA stenosis. The flux proximally (Fluxp) at the point of maximal narrowing must equal the flux distally (Fluxd) along the normal vessel lumen.
To state this mathematically, 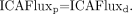
Since flux is equal to the flow of blood passing through a defined cross-sectional area per unit time, the ICA flux is equal to the product of flow velocity and the luminal cross-sectional area. We may therefore substitute, 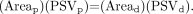
Assuming that the cross-sectional area of the ICA approximates the area of a circle, we may also substitute, 
Rearranging algebraically, we derive, 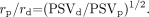
According to NASCET guidelines for the angiographic measurement of ICA stenosis, the luminal diameter, D, is measured proximally at the point of maximal stenosis and distally at a point where the ICA lumen becomes normal; the resultant percentage stenosis is expressed as, 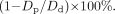
Recognizing that cross-sectional diameter is equal to twice the radius, 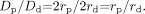
Therefore, by substitution, 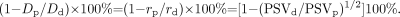
Thus, we have derived a new Doppler sonographic parameter, herein referred to as the SNI, according to the principle of mass balance, in a way that mirrors NASCET methodology for the angiographic determination of ICA stenosis.
For each carotid bifurcation included in our study, values for the SNI were obtained by using the following equation: 
In this equation, the measured velocity originally reported as the PSV was used for PSVp, whereas the value recorded as the highest angle-adjusted velocity observed within the ipsilateral distal ICA was used for PSVd, to calculate each SNI value.
Regression Model
We used the standard model of linear regression, assuming that there is a dependent variable, Y, which in this case is the measured digital subtraction angiographic stenosis, and an independent variable, X, which in this case is the measured Doppler parameter from which Y is to be predicted.
Output data and figures for this linear regression analysis were generated by using the Excel software package (Microsoft Corporation, Redmond, WA). Regression lines for both the PSV values and the SNI values were plotted against the measured angiographic stenosis values (Figs 2 and 3). The values for PSV and SNI that corresponded to 70% angiographic stenosis were determined from the linear regression plots. The accuracy of SNI in predicting 70% or greater angiographic stenosis was compared with that of PSV by using these threshold values.
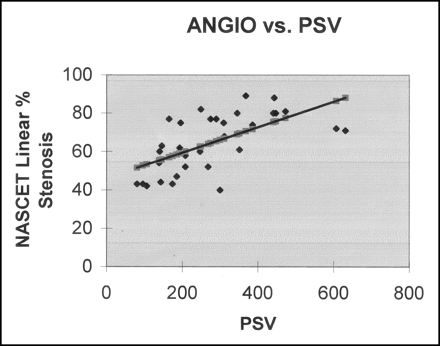
Linear regression plot of PSV versus measured NASCET linear percentage angiographic (ANGIO) stenosis (r2 = 0.38).
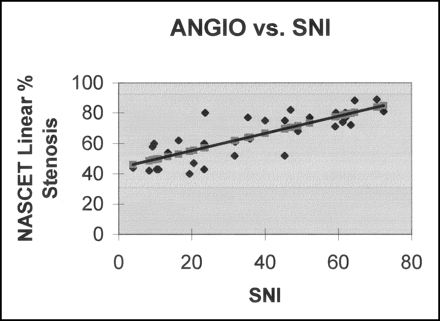
Linear regression plot of SNI versus measured NASCET linear percentage angiographic (ANGIO) stenosis (r2 = 0.64).
A second set of threshold values was also obtained by using a PSV value of 250 cm/s, which was the value in clinical use at our institution for most of the study duration to identify significant carotid stenosis (see Discussion). Using the linear regression plots, the angiographic stenosis value corresponding to a PSV of 250 cm/s on Figure 2 was then used to “read off” the corresponding SNI value from Figure 3. Sensitivity, specificity, and accuracy tables for PSV and SNI at these two different Doppler thresholds were then calculated.
It is noted that both sets of threshold values were chosen prospectively after the linear regression analysis, but before any calculations of sensitivity, specificity, or accuracy, and hence were not chosen retrospectively to enhance the performance of the SNI. No other threshold values were evaluated.
Results
A total of 32 carotid bifurcations were included in the study, with NASCET-style digital subtraction angiographic measurements of linear percentage stenosis ranging from 40% to 89%. Sonographic PSV measurements ranged from 80 to 631 cm/s. Distal ICA velocities ranged from 32 to 201 cm/s. SNI values that were calculated by using the described methodology ranged from 3.9 to 72.4 (unitless parameter).
Statistical Analysis
Linear regression analysis showed a better correlation between SNI and measured NASCET linear percentage angiographic stenosis (r2 = 0.64) as compared with that between PSV and measured NASCET linear percentage angiographic stenosis (r2 = 0.38) (Figs 2 and 3).
By using the data in Figure 2 and the associated linear regression equation of angiographic stenosis versus PSV, the value of PSV that corresponded to a NASCET linear percentage angiographic stenosis of 70% was determined to be 345 cm/s. Similarly, by using the data in Figure 3 and the associated linear regression equation of angiographic stenosis versus SNI, the SNI value that corresponded to a NASCET linear percentage angiographic stenosis of 70% was determined to be 45.5. This set of parameters formed one set of threshold values for comparison of PSV and SNI. The relevant data are in Table 1.
Accuracy of PSV and SNI in identifying significant (NASCET ≥ 70%) stenoses at a Doppler PSV threshold of 345 cm/s
Of the 32 carotid arteries in this study, 15 had a measured NASCET linear percentage angiographic stenosis of 70% or greater, whereas 17 had a stenosis of less than 70%. In the 70% or greater group, nine of 15 stenoses were correctly identified by the PSV threshold of 345 cm/s, whereas 12 of 15 were correctly identified by the corresponding SNI threshold of 45.5. Both PSV and SNI criteria showed a true-negative rate of 16 of 17 in the less than 70% group (Table 1).
A second comparison between the PSV and SNI criteria was undertaken at a lower PSV threshold of 250 cm/s (see Discussion). The SNI value that corresponded to this PSV threshold was 33. This was obtained by first using the PSV versus angiographic stenosis linear regression equation to identify the degree of angiographic stenosis corresponding to a PSV of 250 cm/s in our data set. This value of angiographic stenosis was then used in the SNI versus angiographic stenosis linear regression equation to identify the corresponding SNI value. The relevant data for this set of threshold values are presented in Table 2.
Accuracy of PSV and SNI in identifying significant (NASCET ≥ 70%) stenoses at a Doppler PSV threshold of 250 cm/s
In the 70% or greater group, 13 of 15 stenoses were correctly identified by the PSV threshold of 250 cm/s, whereas 14 of 15 were correctly identified by the corresponding SNI threshold of 33. PSV criteria showed a true-negative rate of 13 of 17, whereas SNI criteria showed a true-negative rate of 14 of 17 in the less than 70% group (Table 2).
Comparing a PSV threshold of 345 cm/s to the corresponding SNI value of 45.5 for identification of angiographic stenosis of 70% or greater, the sensitivity, specificity, and overall accuracy of Doppler sonography were 60% vs. 80%, 94% vs. 94%, and 78% vs. 88%, respectively. Thus, the SNI showed both a greater sensitivity and higher overall accuracy than those of PSV for this set of threshold values.
Comparing a PSV threshold of 250 cm/s and the corresponding SNI value of 33 for prediction of angiographic stenosis of 70% or greater, the sensitivity, specificity, and overall accuracy of Doppler sonography were 87% vs. 93%, 76% vs. 82%, and 81% vs. 88%, respectively. By using this set of threshold values, the SNI showed higher sensitivity, specificity, and accuracy when compared with those of PSV.
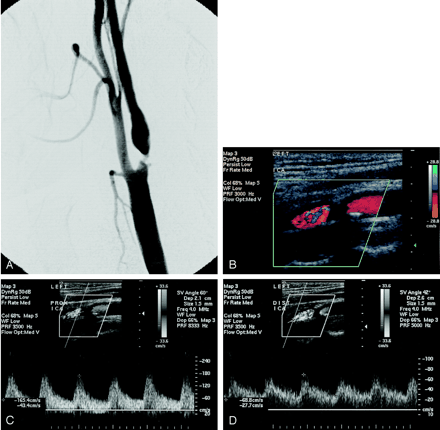
As an example of the improved sensitivity of SNI, we present the data of a symptomatic patient who had a left ICA angiographic stenosis of 77% (Fig 4A). On the day before conventional angiography, this patient underwent bilateral carotid sonography (Fig 4B–D) wherein the left ICA PSV was measured to be 165 cm/s, classifying this patients as having only a moderate stenosis based on the Doppler PSV threshold of 250 cm/s. The calculated SNI value of 35.3 exceeds the corresponding SNI threshold of 33, correctly identifying this stenosis as being 70% or greater.
A, Left ICA angiogram (lateral projection) shows 77% stenosis of the proximal ICA by NASCET criteria, with a small plaque ulceration.
B–D, Color (B) and spectral Doppler images of the same proximal (C) and distal (D) ICA obtained 1 day before conventional angiography.
By using the higher PSV threshold of 345 cm/s, which is similar to the higher threshold value of 325 cm/s (favoring accuracy over sensitivity) published by Moneta et al (5), our database showed that six of 15 patients with high-grade stenosis would be misclassified as having a less than 70% stenosis by using the conventional PSV measurements. Four of these six patients misclassified by PSV alone had SNI values above the corresponding SNI threshold of 45.5, indicating a greater sensitivity of SNI even at high threshold values.
Discussion
During the past 2 decades, numerous different sonographic parameters for the identification of hemodynamically significant carotid stenosis (such as PSV, EDV, and the VICA/VCCA ratio), as well as different thresholds for each of these parameters, have been published (1–15, 18). In an attempt to establish a more universal set of standards, the SRU consensus guidelines for the diagnosis of carotid artery stenosis by using vascular sonography have recently been disseminated (18). In addition to recommending a general protocol for the performance of ICA examinations, the panel recommends stratification of the degree of stenosis as determined by sonography into categories that match those used by the NASCET investigators: no stenosis, less than 50% stenosis, 50–69% stenosis, 70% or greater stenosis, near occlusion, and total occlusion. This decision was clearly made to facilitate the use of carotid sonographic evaluations in clinical decision making according to published NASCET data (23, 27–29). The consensus panel also recommends reliance on PSV as the primary Doppler parameter used in the diagnosis and grading of ICA stenosis.
Nonetheless, the utility of these various Doppler parameters in the detection and grading of carotid stenosis is largely determined by the numeric thresholds selected for their interpretation. These thresholds may be selected according to the presence or absence of symptoms, the desired levels of stenosis prediction (e.g., ≥70% stenosis for symptomatic patients), and the desired levels of accuracy, sensitivity and specificity. Furthermore, the ability of Doppler sonography to substratify angiographic stenoses within the recommended subgroups (e.g., within the 50–69% group) through measurement of PSV has been shown to be limited (21).
It has been suggested that one reason for the limited correlation of Doppler sonography in substratifying patients when compared with cerebral angiography is that the two methodologies of stenosis measurement rely on different anatomic landmarks of internal reference: NASCET guidelines for angiographic diagnosis rely on the normal distal ipsilateral ICA lumen as an internal standard against which the degree of narrowing may be judged, whereas sonography relies on either a single velocity measurement in the proximal ICA (such as PSV and EDV) or on a velocity ratio (such as the VICA/VCCA ratio) by using the distal CCA for comparison rather than the normal distal ICA as in the NASCET methodology. This difference reflects the fact that these Doppler parameters were originally developed independent of the NASCET methodology. After the wide acceptance of NASCET results, Doppler sonography proceeded to fit threshold values to various sets of data to optimize agreement with NASCET-style angiographic measurements, rather than developing brand new sonographic parameters more consistent with the NASCET methodology. Our pilot study was intended to derive and initially evaluate such a new parameter, the SNI.
Since the velocity within a given vessel may vary significantly along its course, it has generally become routine that velocity measurements be obtained at three different points along the ICA, designated as proximal, middle, and distal based on their location relative to the bulb. (Since the location of the carotid bifurcation with respect to the angle of the mandible is variable among patients, the ability to interrogate velocities along the more distal segments of the ICA is also variable.) The highest angle-adjusted velocity observed among the proximal, middle, and distal measurements is then generally taken to represent the PSV within the ICA. Since most ICA stenoses arise at or near the bifurcation, it has been our experience that the proximal or middle ICA velocity measurements are more frequently elevated with respect to the distal velocity and are therefore more often taken to represent the PSV within the vessel. Although the distal ICA velocity is routinely observed, this measurement is only sporadically used in clinical practice.
Previous attempts to improve on the diagnostic accuracy of Doppler sonography by incorporating flow velocity information from the distal ICA have been made. The simple ratio of ICA PSV to distal ipsilateral ICA systolic velocity (called the ICSV/DICSV ratio) has been evaluated prospectively in the assessment of carotid stenosis, by using angiography as the reference standard. In comparison with PSV, the ICSV/DICSV ratio showed better correlation with angiographic stenosis for identifying stenoses of 60% or greater and 70% or greater in vessels with PSV of 100 cm/s or greater (26). Theoretic advantages of this technique include reduction in the possibility of stenosis overestimation due to compensatory increased flow across the stenosis, or from flow diversion to the external carotid artery. In practice, measurement of flow velocity within the distal ICA may occasionally be technically challenging, although technical failure occurs in a small minority of patients. Although data regarding the technical failure rate could not be obtained retrospectively for the purposes of our study, it should be noted that Soulez et al (26) reported a 7.9% technical failure rate by using the strict criteria that DICSV must be measured at least 4 cm distal to the site of PSV measurement and in an area of laminar flow (based on comparison of velocity bandwidth to that of the ipsilateral CCA or the contralateral, nonstenotic ICA).
Our investigation suggests that perhaps the noninvasive detection of critical stenosis and the quantification of stenosis may be further improved in comparison with angiographic measurements through a mathematic incorporation of NASCET principles of measurement. The SNI is derived to specifically mirror the NASCET stenosis measurement by using the assumption of mass balance. Therefore, it is theoretically superior to the traditional Doppler parameters of PSV or simple ICA/CCA velocity ratios, which do not particularly correlate with the NASCET measurement of comparing the diseased site to the normal distal ICA. Moreover, since the SNI is derived to specifically reflect the NASCET stenosis ratio, it is, at least theoretically, also more accurate than the method of Soulez et al (26), which used only the distal ICA flow velocity in a simple ratio.
Although our preliminary investigation suggests that the SNI may be a more optimal sonographic parameter than those currently in use vis-à-vis NASCET angiographic stenosis evaluation, ultimately, like any sonographic parameter, its utility will depend on well-chosen threshold values. In our study, we prospectively chose two sets of threshold values to compare the SNI to the PSV. The first of these was derived from the regression analysis of PSV versus measured angiographic stenosis, by using the PSV value (345 cm/s) corresponding to a stenosis of 70% on the regression line. The value for PSV thus obtained was similar to that of 325 cm/s reported by Moneta et al (7) in a study of various Doppler parameters that were selected to maximize the overall accuracy of predicting stenosis of 70% or greater. However, this value is higher than that used in most laboratories, including our own, and may lead to diminished sensitivity. The second PSV threshold chosen was 250 cm/s, which was the threshold value for high-grade stenosis used in our vascular laboratory during most the study time. It is nearly identical with the values of 270 cm/s proposed by Neale et al (6) derived with special reference to the NASCET data, and 230 cm/s proposed by Huston et al (10). It is also the value used in a new criteria recently proposed by Berland and Weber (30) for diagnosing 70–99% stenosis.
In the attempt to develop a new sonographic parameter more closely correlated to the NASCET methodology of measurement, several important ancillary issues deserve further comment. The first of these is regarding the use of sonographic planimetry for direct stenosis measurement, by measuring either the linear vessel diameter or vessel cross-sectional area in the proximal ICA and the distal ICA to directly calculate the degree of stenosis. Certainly, such an approach would correlate more closely with NASCET methodology than anything else. However, various authors have indicated that such measurements, especially in high-grade stenosis, are often difficult to perform and do not reliably correlate to angiography (30–31). Reasons for this include heavy calcification in the area of greatest stenosis, inability to accurately measure vessel borders, and lack of accuracy of longitudinal plane measurements of carotid lumen. Therefore, it has been widely accepted that physiologic assessment of flow velocity as a reflection of carotid stenosis is the more accurate sonographic method, until critical stenoses with near-occlusion are reached.
Although, to our knowledge, the SNI is the sonographic parameter most closely correlated to the NASCET methodology thus far, it is important to note that it still relies on velocity measurements at a single time point (the point of PSV). Therefore, there is still a lack of a simple correlation between the SNI and measured angiographic stenosis, with no straightforward way available to provide a “conversion table” from one to the other. This is because there are several other considerations not accounted for in the derivation presented, which is intentionally kept simple so that the SNI may be readily used in the vascular laboratory. Attempting to fully characterize the complex biologic compensations that occur in the face of significant stenosis with a single PSV measurement is quite difficult. Certainly, as a vessel becomes narrower, velocity must increase to maintain flux, which is the crux of our mass-balance argument. However, the true mass-balance equations stipulate that bulk flow into the carotid equals bulk flow out. Thus, in reality, we must take into account the entire velocity profile over a cardiac cycle, or a defined unit of time, not just a single PSV measurement. Thus, 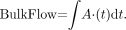
In this equation, A represents the cross-sectional area of the vessel, whereas v represents the flow velocity within it. Calculating true bulk flow would require integrating over the entire velocity-time curve for a given period of time, such as one cardiac cycle. If there are changes in the Doppler waveform between the stenotic proximal ICA and the distal normal ICA (e.g., a greater distal diastolic flow component in the setting of tighter stenoses), then we are dealing with a more complex phenomenon than can be captured by measuring a single PSV value proximally and distally. To further complicate matters, the cross-sectional area A is actually also a function of time, A(t), varying with cardiac pulsations.
A final and potentially serious limitation of the SNI has to do with the evaluation of near occlusion in the setting of a highly stenotic ICA. The SNI, of course, still relies on velocity measurements to assess the degree of luminal narrowing. However, it is known that velocity-based sonographic measurements become unreliable in this setting, and gray-scale evaluation along with color or power Doppler of the ICA are the preferred modes of sonographic evaluation (18, 30). In our preliminary study, no attempt was made to evaluate the accuracy of the SNI in the setting of near occlusion. Since velocity alterations, including velocity normalization or decrease in the setting of critical stenosis, would affect both the proximal and the distal ICA, the SNI may function better than PSV, EDV, or the VICA/VCCA ratio. However, this issue will require further separate study.
Overall, although the SNI demonstrated increased sensitivity, specificity, and accuracy in this pilot study, we underscore that it would be premature to base surgical decision making solely on the SNI. We also note that at our institution, surgical decisions continue to be made primarily by using a combination of MR angiography and sonography and assessing for a concordance between these two modalities, as this has been shown to be superior to either technique alone (32).
However, the SNI makes a serious attempt at tackling the issue of an essentially independent development of the NASCET-style measurement protocols and the sonographic criteria for significant stenosis, with an almost retrospective attempt to fit together two disjointed measurement methods. The former is obviously based on an anatomic measurement, whereas the latter is physiologic, and it is unlikely that a perfect match can ever be achieved.
Conclusion
We have shown that the incorporation of distal ICA flow velocity information in a rigorous mathematical fashion based on the principle of bulk flow mass balance improves the diagnostic accuracy of the most widely used and most accurate Doppler sonographic parameter, the PSV. We believe this is because the SNI is derived to specifically mirror the NASCET methodology of stenosis measurement. Confirmation of this hypothesis through a larger, prospective study is needed.
References
- Received February 4, 2004.
- Accepted after revision April 5, 2004.
- Copyright © American Society of Neuroradiology