Abstract
BACKGROUND AND PURPOSE: Endovascular coil embolization is used increasingly to treat cerebral aneurysms. The purpose of our study was to quantify the incidence of CT-detectable abnormalities after aneurysm coiling and map the radiographic and clinical progression.
MATERIALS AND METHODS: We reviewed the radiographic and clinical sequelae of 30 consecutive patients with aneurysms who underwent endosaccular coiling followed by head CT scans. Patients with CT abnormalities received follow-up scans at 4 to 6 hours and 20 to 25 hours. Contrast enhancement was defined as CT hyperdensities with progressive resolution over 25 hours and a Hounsfield unit (HU) of less than 70. The incidence of CT abnormalities was recorded and correlated with amount of contrast used, use of antiplatelet agents, procedure time, and clinical sequelae.
RESULTS: Seven patients (23%) had new hyperdensities on CT scan. Four showed gyral hyperattenuation; 1 showed basal ganglia hyperattenuation, and 2 showed a combination of these patterns. All were asymptomatic and were consistent with contrast enhancement, with complete resolution in 5 of 7 and partial resolution in 2 of 7 by 20 to 25 hours. Antithrombotic or antiplatelet medication was continued in all cases. The amount of contrast used (P = .014) and the use of antiplatelet medication (P = .029) were statistically correlated with the presence of hyperattenuation after aneurysm coiling, whereas the length of the procedure was not (P = .162).
CONCLUSION: Contrast enhancement, unlike contrast extravasation, is a fairly common and clinically benign finding after aneurysm coiling. The enhancement resolves by 25 hours in most cases, regardless of the continuation of antithrombotic or antiplatelet therapy.
Endovascular embolization of intracranial aneurysms (IA) with coils has become an increasingly accepted technique of therapy that is now the preferred method of treating IAs in some centers.1 Although the safety and efficacy of this technique are now well documented, as the technology continues to grow additional questions arise. Newer devices, in particular, the Neuroform stent (Boston Scientific, Natick, Mass), have facilitated the treatment of wide-necked aneurysms but require the use of potent antiplatelet agents such as aspirin and clopidogrel. Increased safety of coiling may be attributed to the use of prophylactic agents against thrombus formation, such as heparin, aspirin, clopidogrel, and eptifibatide,2 and the use of successful treatments for thromboembolic phenomena, namely abciximab, heparin, clopidogrel, eptifibatide, and aspirin.3
Few studies on the results of imaging in the acute period after coiling have been published,4–7 and only 1 other report has looked at a consecutive series of patients who underwent head CT scans immediately after coiling.4 In contrast to the report in which CT scans were not performed beyond 4 to 6 hours postprocedure, we have extended the imaging evaluation to 20 to 25 hours. Given the increased use of antithrombotic and antiplatelet agents, it would be important to know the rate of perioperative hemorrhage or other CT-scan abnormalities, symptomatic or not, such that decisions can be made regarding continuation of such medication. This is the first paper to study the correlation of antiplatelet use with new CT findings after coiling and to address whether continued use is safe when a new abnormality is encountered. We reviewed our series of 30 consecutively treated aneurysms by using Guglielmi detachable coils (GDC; Boston Scientific) or Neuroform stent-assisted coiling and a protocol of perioperative CT scan imaging.
Patients and Techniques
Procedural Protocol
The Institutional Review Board granted us permission to review the charts of 30 consecutive patients who had undergone endovascular coiling of intracranial aneurysms at the New Jersey Neuroscience Institute. All coiling procedures were performed by the senior author (J.L.B.), and all patients underwent head CT scanning without contrast immediately after the procedure. If the CT scan showed evidence for either a hemorrhage or other hyperattenuation (other than the artifact related to the coils or to previously identified intracranial blood in the case of patients presenting with hemorrhage), repeat CT scanning was obtained in 4 to 6 hours. If that CT scan continued to show the new abnormality, a third scan was obtained at 20 to 25 hours.
CT Scanning and Interpretation
All patients underwent head CT scanning using a LightSpeed Pro16 (GE Healthcare, Milwaukee, Wis) with a 5-mm section thickness and sections from the vertex through the foramen magnum. Each CT scan was reviewed by a neuroradiologist, and abnormalities were recorded. The neuroradiologist was not blinded to the clinical history. Areas of new focal increased attenuation were individually selected by the radiologist, and the HU was measured. Hyperdensities in which the measured HU were greater than 70 and which persisted at 20 to 25 hours after the procedure were interpreted as hemorrhage and those less than 70 and that showed progressive resolution for the 20- to 25-hour period after the procedure as contrast enhancement hyperattenuation (CEH). This definition of CEH represents a more conservative threshold than that described in conjunction with CT findings after intra-arterial thrombolysis for acute stroke (HU <90).8,9
Data Collection and Analysis
Data collected on the study group included age; sex; presentation; aneurysm location and size; amount of contrast used (mL/kg) for the procedure; duration (minutes) of the procedure; number of coils deployed; whether a stent was used; location of the hyperattenuation if found; HU of the hyperattenuation at 0 hours, 4 to 6 hours, and 20 to 25 hours; use of heparin, aspirin, clopidogrel, and abciximab; and clinical examination at all time points. We performed statistical analysis using the Student t test and Fisher exact test with significance defined as P < .05.
Endovascular Coiling
Coiling with use of a biplane fluoroscopic machine with 3D rotational capability was performed with the patients under general anesthesia. Iodixanol 320 mgI/mL was the contrast agent used in all patients. Most of the contrast was delivered into the vascular territory supplying the target aneurysm. However, all patients underwent 4-vessel angiography at the time of treatment to identify any vascular abnormalities and to assess collateral circulation. After the 3D angiography was used to select the best working image for coiling and to measure the aneurysmal neck and fundus, a 6F guiding catheter was placed into the cervical region of the vessel of interest, and multiple high-magnification roadmap hand injection angiograms were obtained. GDC coils were used in all instances, with the goal to achieve complete obliteration of the aneurysm when possible. Heparin was used in all instances and was given immediately after sheath placement in unruptured lesions and after the first coil was deployed in ruptured aneurysms; activated clotting time of 2 to 2.5 times the baseline value was the goal in all cases. Heparin was continued for 12 to 48 hours after the procedure. Patients in whom Neuroform stents were used were premedicated with clopidogrel (75 mg daily) and aspirin (325 mg daily) for 5 days before the procedure in unruptured aneurysms and were loaded with clopidogrel (300 mg via orogastric tube) and aspirin (300 mg rectally) for ruptured aneurysms. Some patients with unruptured aneurysms in whom stents were not placed received aspirin (325 mg orally) on the day of the coiling before anesthesia.
Results
Preoperative Data
Of the 30 patients evaluated, there were 23 women and 7 men with ages ranging from 32 to 80 years (mean age, 57 years). Sixteen patients presented with hemorrhage. Four patients underwent stent-assisted coiling. A total of 28 patients had aneurysms in the anterior circulation with aneurysmal size ranging from 2.5 to 22 mm (mean, 7.1 mm). Two patients were premedicated with clopidogrel or aspirin for 5 days, 3 patients received aspirin as their baseline medication preoperatively, and 13 patients received aspirin the day of the procedure (8 [unruptured] the morning of the procedure and 5 [subarachnoid hemorrhage] intraoperatively). Eleven patients were taking no antiplatelet agents perioperatively. In addition, 6 patients received intra-arterial or intravenous abciximab, all but 1 of whom was also medicated with aspirin or clopidogrel, or both, in the perioperative period.
CT scans were performed on all patients with CEH at 3 time points except for 1 patient who did not receive a CT scan at the 20- to 25-hour mark. There were 7 (23%) of 30 patients evaluated who were found to have CEH immediately after coiling. Their clinical presentation, procedural details, and perioperative findings are summarized in Table 1. There were 5 women and 2 men with ages ranging from 42 to 80 years old (mean age, 60 years). Only one of the patients had presented with SAH. Six of the 7 patients had aneurysms in the anterior circulation. All 7 patients showed progressive resolution of the degree of hyperattenuation with time. Complete resolution was noted in 5 patients at 20 to 25 hours, and partial resolution was seen in 2 patients. Data were not available in 1 patient at the 20- to 25-hour time period. No patients had hyperdensities interpreted as new hemorrhages on their posttreatment CT scans.
Patient data of cases with postprocedural CT hyperdensities
Illustrative Cases
Patient 2
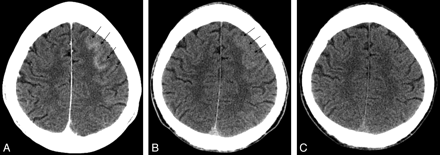
This 43-year-old man presented to the emergency department with a sudden onset of severe headache. The results of his CT scan and lumbar puncture were both negative for hemorrhage. He underwent cerebral angiography with intention to treat after a CT angiogram revealed a 6-mm anterior communicating artery (AComA) region aneurysm. The aneurysm was coiled with the guiding catheter in the left internal carotid artery. During coiling, a small amount of coil herniation into the AComA prompted the use of aspirin and clopidogrel. CT scan immediately after coiling showed a small amount of CEH in the left frontal gyral region (Fig 1A). The CEH resolved the next day (Fig 1B, C), and the patient remained neurologically intact. He was continued on his clopidogrel and aspirin and was discharged on these medications.
A, immediate postembolization noncontrast head CT shows a gyral pattern of enhancement (arrows). B, C, interval resolution of enhancement at 6 hours (B) and 24 hours (C), respectively (arrows).
Patient 3
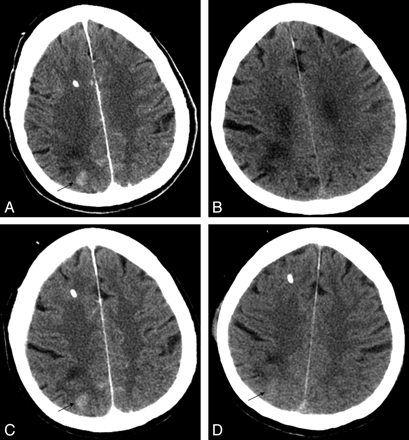
This patient presented with generalized headaches and was found to have an unruptured 8-mm AComA region aneurysm. The patient underwent endovascular coiling of the aneurysm with the guiding catheter in the left internal carotid artery. At the end of the embolization, the contralateral anterior cerebral artery (ACA) was found to have markedly less filling. This prompted the use of intra-arterial and intravenous abciximab, which resulted in reopening of the right ACA. The immediate postoperative CT scan showed CEH in the left basal ganglia as well as in the left frontal gyri (Fig 2A, B). The CEH completely resolved the next day (Fig 2C–F), despite being maintained on a 12-hour intravenous infusion of abciximab, and oral clopidogrel and aspirin. The patient experienced very mild sensory changes and weakness of his right leg; he ultimately recovered to his baseline preoperative neurologic status 2 weeks after the procedure.
A, B, immediate postembolization noncontrast head CT shows left caudate (A) and gyral (B) enhancement (arrows). C, D, interval improvement of caudate (C) and gyral (D) enhancement at 6 hours (arrows). E, F, resolution of caudate (E) and gyral (F) enhancement at 24 hours.
Patient 5
This 80-year-old woman presented with a severe headache and collapsed. She was brought to the emergency department and was found to be confused and lethargic. A brain CT scan without contrast showed a diffuse subarachnoid hemorrhage. Cerebral angiography revealed a small (4.5 mm) basilar terminus aneurysm that had a wide neck. A decision was made to proceed with Neuroform stent-assisted coiling. She was loaded with clopidogrel (300 mg) and aspirin (325 mg) via orogastric tube while in the angiography suite. A Neuroform stent was placed across the aneurysmal neck into the right posterior cerebral artery and the basilar artery. The aneurysm was then successfully coiled. Immediate postoperative CT scan showed a small area of CEH in the right occipital lobe (Fig 3A), not present in the initial scan (Fig 3B). The abnormal finding showed progressive resolution over successive scans (Fig 3C–D). After the coiling procedure, she awoke without any new deficits but eventually deteriorated from vasospasm; the family decided to withdraw care given her poor neurologic condition.
A, immediate postembolization noncontrast head CT shows a gyral pattern of enhancement (arrow). B, noncontrast head CT obtained at presentation without gyral subarachnoid blood in the area of postcoiling enhancement. C,D, interval resolution of enhancement at 6 hours (C) and 24 hours (D), respectively (arrows).
Comparing the CEH+ and CEH− Groups
Volume of contrast used was available in 27 of 30 procedures (Table 2). The amount of contrast used in the CEH+ group ranged from 2.56 to 7.76 mL/kg (mean 5.74 mL/kg) compared with the CEH− group, which ranged from 0.69 to 7.69 mL/kg (mean, 3.63 mL/kg). The difference between these groups was significant (P = .014). Procedural time of the CEH+ group was 102 to 360 minutes (mean, 221 minutes) compared with the CEH− group, which was 41 to 356 minutes (mean, 171 minutes). This was not significant (P = .162). An antiplatelet agent (aspirin, clopidogrel, or abciximab) was used in 7 of 7 patients with CEH+ and in 12 of 23 patients with CEH− (P = .029).
Procedure data on treated patients
Discussion
The main finding of this paper was that contrast enhancement on a head CT scan is a frequent finding after endosaccular coil embolization of cerebral aneurysms, occurring in 23% of our consecutively evaluated series of 30 treated aneurysms. This result was not associated with neurologic symptoms and resolved radiographically (completely in 5/7 and partially in 2/7) by 20 to 25 hours. Heparin and antiplatelet agents including aspirin, clopidogrel, and abciximab were continued in all cases and did not prevent the radiographic resolution of the enhancement and did not lead to neurologic change in any patient. In this series, this was a benign finding and should not be confused with contrast extravasation or hemorrhage, which would not be expected to resolve as quickly and should have a HU measurement greater than 70.8,9
Historical Background: Lessons Learned From Diagnostic Angiography and Intra-arterial tPA for Acute Stroke
The initial description of CEH was in association with diagnostic angiography and first reported with the widespread use of CT scanning in the 1970s.10 Its documentation after aortography, coronary angiography,11 as well as selective carotid injection cerebral angiography,12 was believed to be the result of disruption of the blood-brain barrier (BBB) accompanying the use of hyperosmolar ionic contrast medium.12,13
Further evidence for this hypothesized pathophysiology of CEH was the finding of contrast enhancement on CT scan after angiography in patients with intracranial pathology known to disrupt the BBB, such as tumor or stroke. CEH was associated with seizures on several occasions, but rarely was there a persistent neurologic deficit.13 The finding of increased brightness on noncontrast CT scan after intra-arterial thrombolysis for acute stroke has been well described and defined as CEH if the HU is less than 90 and the attenuation resolves by 24 hours.8,9,14–16
CEH After Endovascular Cerebral Aneurysm Treatment
The first description of CEH after coiling of a cerebral aneurysm was in 2004, when this phenomenon was described after coiling of a ruptured AcomA aneurysm in a 72-year-old man.17 The contrast enhancement was found throughout the left hemisphere and basal ganglia on a CT scan obtained 1 hour after coiling and was presumed to be the cause of new aphasia and right hemiparesis in this patient. The authors ascribed this to breakdown of the BBB in response to a large contrast load (260 mL) selectively injected into the left carotid artery. The CT hyperattenuation resolved by 11 hours, and the patient made a complete neurologic recovery.
In the one other reported study looking at CT findings immediately after coiling, CT scans on 49 of 100 consecutively treated aneurysms demonstrated focal hyperdensities.4 All the patients remained asymptomatic, and the hyperattenuation resolved at least partially in all cases on a follow-up scan obtained 4 to 6 hours later. CEH was defined broadly as a new hyperintensity on noncontrast CT scan obtained immediately after coiling. A balloon remodeling technique was used in 74 of 100 patients, and there was a significantly increased rate of hyperattenuation when balloons were used (54%) compared with when they were not (34.6%), prompting the authors to suggest that intermittent contrast trapping by the balloons was related to the physiologic mechanism of this radiographic finding. The authors found that CEH was statistically correlated with contrast load (relative to body weight), microcatheter time, total balloon inflation time, and number of balloon inflations and elapsed time between the end of the procedure and obtaining the CT scan (shorter times led to increased likelihood of CEH). Correlation with antiplatelet use was not evaluated.
Unlike the report by Ozturk et al,4 we did not use the balloon-remodeling technique, and this is perhaps why our incidence was significantly lower (23% vs 49%). Their results in which they did not use balloons is more similar to ours (23% vs 34.6%). Of the 49 aneurysm treatments that demonstrated CEH in the Ozturk study,4 none had hyperattenuation in the deep gray matter as we observed in 3 of 7 patients and as was described in the first reported case by Uchiyama.17 They also performed MR imaging examinations 4 to 6 hours after the procedure, which showed no evidence for infarction in the area of CEH. Patients in whom there was thrombus formation or distal emboli were excluded from their study, whereas 3 of our CEH patients had thromboembolic events treated with antiplatelet agents. The correlation between CEH and antiplatelet use was not evaluated in their study. Lastly, we obtained repeat CT imaging at both 4 to 6 hours and 20 to 25 hours in patients with CEH, whereas Ozturk et al4 did not report on imaging beyond 4 to 6 hours.
In their report, they found a statistical correlation between the amount of contrast used and the presence of CEH, with 4.7 mL/kg as the cutoff for the minimum amount of contrast in which CEH was found. Our study also was able to demonstrate a statistically significant correlation between the contrast load relative to body weight and the presence of CEH. However, 2 patients with CEH+ received contrast loads below the minimum cutoff of 4.7 mL/kg found by Ozturk,4 whereas 5 patients with CEH− received doses above this threshold. This suggests that a high contrast load may be necessary, but not sufficient, for CEH. It is interesting to note that we have also seen CEH after other neuroendovascular procedures (such as embolization of an arteriovenous malformation and intracranial stent placement, data not presented here), which suggests that the causal mechanism is unrelated to the presence of an aneurysm.
Caveats
Despite a correlation between contrast load and CEH, there was no specific dye load that reliably led to CEH. Why some patients experienced CEH whereas others did not, is not clear. It is possible that other patient-specific factors that were not controlled for in this study, such as age, medical comorbidities, clinical condition, presence of subarachnoid hemorrhage, hydrocephalus, vasospasm, or other metabolic derangements that might affect the BBB might explain this. Second, the finding of a statistically significant correlation between the use of an antiplatelet agent and CEH+ is of unclear significance. This result might simply reflect a multifactorial process in which other more important factors associated with antiplatelet use, such as stent placement or an intraprocedural thromboembolic phenomenon, may suggest a direct correlation that is artifactual.
Conclusions
Contrast enhancement in the brain parenchyma after endovascular coiling is a common occurrence. In this small series, CEH seems to undergo progressive radiographic resolution during 25 hours and has no neurologic sequelae, despite continuation of antiplatelet and antithrombotic agents. The enhancement, hypothesized to result from disruption of the BBB, occurs in the cortical gyri, deep gray matter, or both and seems to correlate with the amount of contrast used in the procedure and the use of antiplatelet agents.
Acknowledgments
We gratefully acknowledge the assistance of Barry H. Cohen, PhD, with statistical analysis.
References
- Received June 5, 2007.
- Accepted after revision August 8, 2007.
- Copyright © American Society of Neuroradiology