Abstract
BACKGROUND AND PURPOSE: Combining percutaneous plasma-mediated radio-frequency (pmRF) ablation with vertebral body augmentation offers an alternative treatment to surgical intervention options for advanced metastatic spinal lesions and is particularly useful for cases with cortical destruction and/or epidural extension. This study evaluates bone cement deposition patterns and extravasation in treated vertebral bodies in relation to the metastatic lesion after using this combined approach.
MATERIALS AND METHODS: Retrospective assessments of CT images performed before/after the procedures were evaluated in 37 patients (44 levels) with advanced metastatic lesions. A void was created in the anterior portion of the tumor-infiltrated vertebral body by using a bipolar plasma-mediated radio-frequency−based wand, followed by deposition of bone cement. Pain measured by visual analog scale score was recorded preprocedure and 2–4 weeks afterward.
RESULTS: In 19 (43%) levels, 90%–100% of the cement was deposited in the anterior two thirds of the vertebral body. In 34 levels (77%), 75% or more of the cement was deposited in the anterior two thirds of the vertebral body. In 13/15 (86%) levels with posterior lesions, cement was deposited anterior to the lesion. No extravasation was observed in 13 levels (29.5%). Two clinically insignificant incidences of epidural extravasation were noted. Pain relief after the procedure was reported by 25/28 (89.5%) patients with available data.
CONCLUSIONS: pmRF ablation may allow greater cement-deposition control, increasing the likelihood of successfully stabilizing the anterior two thirds of the vertebral body. This combined technique appeared particularly useful in cases with posteriorly located lesions. The incidence of cement extravasation was relatively high but clinically insignificant.
Though percutaneous cement injection has been widely used in vertebroplasty and kyphoplasty procedures to treat vertebral compression fractures (VCF), these treatments are associated with higher complication rates in patients with metastatic spine lesions than in those with benign osteoporotic fractures.1 Advanced metastatic spine lesions—those with epidural extension, cortical disruption, paraspinal extension, or combinations thereof—are considered relative contraindications for conventional vertebroplasty or kyphoplasty. Recently, a new technique has been introduced to perform cement augmentation in patients with advanced lesions.
Using plasma-mediated radio-frequency (pmRF) ablation, one can create a cavity in the anterior part of the vertebral body before cement augmentation.2 The pmRF ablation process uses highly precise molecular dissociation,3–5 minimizing thermal damage to neighboring spinal tissues,6 compared with higher temperature conventional electrosurgical methods.
The resultant tissue void is posited to allow more control over cement deposition during injection and may help direct cement away from a compromised posterior cortex or epidural extension. Prospective review of patients undergoing the procedure showed the technique to be clinically feasible and associated with pain reduction.
The ability to reliably deposit cement in the anterior two thirds of the vertebral body in patients with advanced metastatic lesions by using this technique could potentially alter surgical management for these cases. Patients otherwise deemed untreatable or high risk by using traditional methods may find a safe and viable treatment option in the combination of pmRF ablation and bone cement augmentation. Stabilizing the spine quickly and safely is of great importance for these patients because vertebral compression fractures can significantly increase health complications, number of hospitalizations, and overall mortality.7,8
This study evaluated bone cement deposition patterns and extravasation in treated vertebral bodies by examining CT images. The study aimed to answer the following questions: 1) Is the bone cement reliably placed into the anterior two-thirds portion of the vertebral body? 2) What is the relationship between the cement-deposition patterns and lesion locations? 3) What is the pattern and incidence of cement extravasation?
Materials and Methods
Medical charts from 37 patients (44 levels, 21 women and 16 men) consecutively treated for painful VCF due to metastatic lesions were retrospectively reviewed and included in this study. Institutional review board approval was obtained before beginning the review. Lesions were treated in vertebral bodies in the lower thoracic region (T6 and below) and lumbar spine. All vertebral bodies demonstrated cortical disruption, epidural extension, paraspinal extension, or a combination of these findings. All patients except 1 underwent MR imaging before the procedure to determine the extent of the epidural disease. The patient who did not undergo MR imaging underwent a bone scanning instead. CT examination was also performed on all patients before and immediately following cement augmentation. All except 1 of the postprocedure examinations (Brilliance CT scanner; Philips Medical Systems, Best, the Netherlands) had sagittal and coronal reconstruction images. Preprocedure CT studies were evaluated for lesion location, wall destruction, epidural extension, and the type of lesion (sclerotic or lytic).
Details of the Procedure
The bipolar radio-frequency−based device (Cavity SpineWand; ArthroCare, Sunnyvale, Calif) was advanced into the delivery port (harvesting needle or introducer kit) until its tip protruded beyond the cannula tip. Every effort was made to place the needle tip in the far anterior portion of the vertebral body regardless of the location of the lesion. With the radio-frequency controller placed on a setting of 6, the activated device was directed anteriorly through the malignant mass to ablate (ie, excise) tissue to form a small channel. The curve of the device allows it to ablate slightly beyond the trajectory of the access cannula. This maneuver was repeated along several different clock positions (various orientations) to etch a cavity. Ablation was stopped when a noticeable reduction in tactile resistance was detected. For inserting the device toward the anterior aspect of the vertebral body, the tissue dissolution (ie, coblation) setting was used; for retracting the device posteriorly, the coagulation setting was used to provide hemostasis. A total of 3–6 passes were made to complete the cavity, which took between 30 and 60 seconds, depending on the size of the vertebral body and lesion. The device removes between 1.5 and 2 mL of tissue during this process. Bone cement (Palacos; Zimmer, Warsaw, Ind) and Biotrace sterile barium sulfate (Bryan, Woburn, Mass) were then injected into the ablated cavity under fluoroscopic guidance. On average, 3–6 mL of bone cement was found to be sufficient to fill the ablated vertebral body cavity. This is in keeping with our procedural experience from a prior study.2
Evaluation of cement deposition was performed by using a standard PACS system (ProVision Workstation; Cerner, Kansas City, Mo). An axial cut allowed gross assessment of the deposited cement. With the axial cut that contains the maximum amount of cement, 2 lines were drawn on the image: 1 along the anterior border of the vertebral body and the other along the posterior border (or the presumed posterior border if this was obscured by cortical disruption or epidural extension). The distance between these 2 lines was recorded. Another 2 lines were drawn at equal distance and parallel to the first 2 lines to divide the vertebral body into thirds. The amount of cement present in the anterior two thirds of the vertebral body was visually estimated in increments of 5%, and the estimate was confirmed in the axial and coronal planes. This process was performed on all levels and repeated twice. Discrepancy was present in 4 of the 37 cases; an average was calculated in those cases. Cement deposition in relation to the metastatic lesion was also recorded. Cement extravasation was evaluated in all 3 planes; when it was seen on 1 plane, every effort was made to confirm it on the other planes.
Visual analog scale (VAS) scores were obtained before the procedure and 2–4 weeks after the procedure. Scores were available for 28 of 37 (76%) patients.
Results
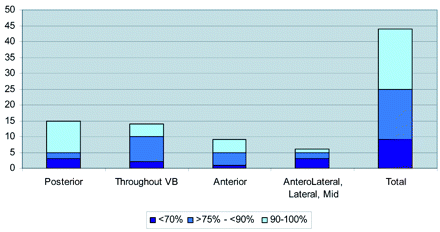
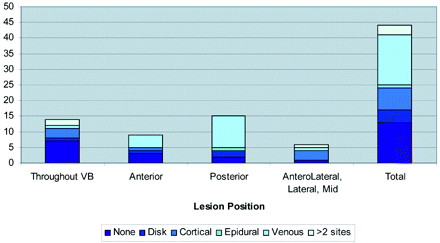
The On-line Table presents a summary of the study population, including age, sex, cancer type, levels treated, the anatomy of the metastatic lesion, preprocedure lesion location, pain scores, and the pattern of cement deposition in relation to the lesions. Figure 1 shows bone cement placement into the anterior vertebral body with respect to lesion position. Figure 2 shows the extravasation pattern with respect to lesion position. Figures 3 and 4 provide pre- and post-procedure CT and MR images of 2 patient cases.
Bone cement placement into the anterior vertebral body (VB) by lesion position.
Extravasation site by position of the lesion. VB indicates vertebral body.
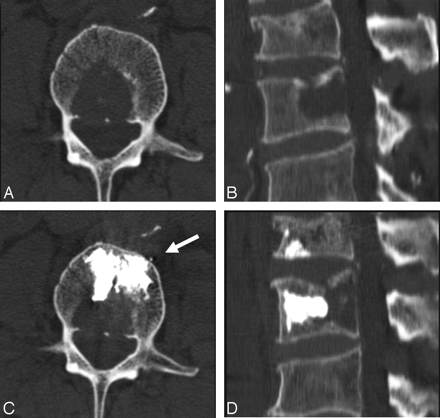
A 83-year-old man with a history of lymphoma. A and B, Axial and sagittal reconstruction CT scans show the lytic lesion in the posterior part of the L2 vertebral body. C and D, The corresponding CT scans obtained immediately after the procedure show all the injected cement in the anterior two thirds of the vertebral body and anterior to the lytic lesion. Arrow shows minimal anterior venous leakage.
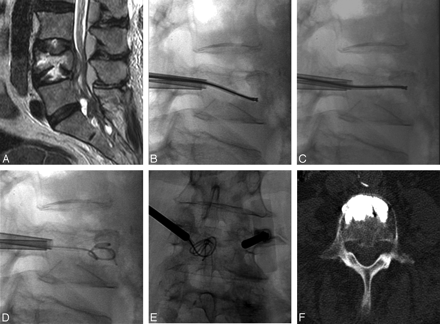
A 71-year-old woman with undifferentiated cancer and a lesion at L4. A, T2-weighted sagittal image shows epidural extension and a metastatic lesion in the spinal canal. B and C, A void is created in the vertebral body by debulking the spinal tumor by using the curved pmRF-based wand before vertebral body augmentation with bone cement. D and E, A 35F angiographic wire is seen coiled inside the created cavity in both anteroposterior and lateral projections. F, Axial CT image after cement deposition reveals excellent anterior placement of bone cement.
Bone Cement Placement into the Anterior Two Thirds of the Vertebral Body
In 19 (43%) levels, 90%–100% of cement was placed into the anterior two-thirds portion of the vertebral body (Fig 1). In 34 (77%) levels, ≥75% of cement was detected in the anterior two thirds of the vertebral body.
Where the lytic lesion was found in the posterior part of the vertebral body (15 levels), cement was seen in the anterior two-thirds portion of the vertebral body (anterior to the lesion) in 13 levels. When the metastatic lesion was found in the anterior portion of the vertebral body (12 levels), injected cement was found in the anterior part in 11 cases. When the lesion was distributed throughout the vertebral body, the tendency was to see most of the bone cement in the anterior two thirds.
Bone Cement Extravasation
A total of 31 levels of extravasation (18 venous [40%], 10 cortical [22%], 5 diskal [11%]) were detected by using CT; all cases were minimal and clinically insignificant. Two small epidural extravasations were also noted; in 1 of these cases, extravasation occurred adjacent to the neural foramen and was associated with clinical symptoms. These were treated by using a selective nerve root block. Extravasation appeared to occur most frequently in vertebral bodies possessing a posterior lesion (Fig 2). No evidence of extravasation was observed in 13 levels (29.5%). Three patients underwent selective nerve root block after the procedure, 2 of whom were symptomatic due to tumor extension into the neural foramina. In only 1 case, radicular symptoms developed postprocedurally and were thought to be due to cement extravasation in relation to the existing nerve. The patient developed temporary pain relief but had no reported follow-up for the radicular symptoms.
Clinical Follow-Up
VAS scores for pain were recorded both immediately before the procedure and 2–4 weeks afterward and were available for 28 of 37 patients. Twenty-five of 28 (89.5%) reported pain relief.
Discussion
Spinal metastases are estimated to develop in 27% of patients with cancer.9 Stabilizing the spine with cement augmentation is a key concern in patients with advanced spinal metastases. For these patients, conventional treatments such as vertebroplasty and kyphoplasty are associated with high complication rates,1,10,11 and many are deemed untreatable through these methods.
Neural compromise or epidural extension associated with conventional vertebroplasty occurs in approximately 10% of patients with spinal metastases, compared with 2%–5% of patients with vertebral angiomas or 1%–3% of patients with osteoporotic lesions.12–16 This is possibly due to destruction of the vertebral cortex, which is estimated by Deramond et al11 to occur in >50% of patients with spinal metastases.
Our study has shown that the novel technique of coupling pmRF ablation with bone cement augmentation is a viable treatment option for patients with advanced metastases, allowing reliable cement deposition in the anterior two thirds of the vertebral body. This can potentially alter the surgical management of these patients by allowing effective reconstruction of the anterior portion of the vertebral body without by using allograft, autograft, cages, or plates, thus avoiding an extensive anterior surgical approach.
The surgical treatment of metastatic lesions depends on the location of the tumor, the presence or absence of spinal instability, and the presence or absence of neural compression or neural deficit. In 1989, James Weinstein17 proposed a model to optimize surgical planning in patients with spinal metastasis. This model delineates the vertebral body into 4 zones, which are used to describe the location of the metastatic lesion. Zone I includes the spinous process to the pars interarticularis and superior facet. Zone II encompasses the superior articular facet, transverse process, and the pedicle from the level of the pars to its junction with the vertebral body. Zone III consists of the anterior three quarters of the vertebral body, and zone IV is the posterior quarter of the vertebral body. Zone I and II lesions are accessed by using a posterior or posterolateral surgical approach. These types of lesions are usually treated with posterior decompression and stabilization. Zone III lesions are typically accessed through an anterior surgical approach. This allows direct access to the tumor, and effective reconstruction of the weight-bearing anterior portion of the spinal column can be achieved by using an allograft, an autograft, cages, plates, or polymethylmethacrylate (PMMA) implants.18 Zone IV lesions are the most difficult to treat because they require a combined anterior and posterior approach. Because of the precision of our technique in depositing bone cement into the anterior two thirds of the vertebral body, it seems most likely to influence the surgical management of patients with zone III and IV lesions.
pmRF ablation operates by using radio-frequency energy to excite the electrolytes of a conductive medium, such as saline solution, to create a precisely focused plasma field. The energized particles in the plasma field have sufficient energy to break molecular bonds, excising or dissolving soft tissue at relatively low temperatures (40°–70°C). This generates far less heat in surrounding tissue than the conventional diathermy mechanism,19 complementing the precision of the initial excision process by minimizing thermal damage and necrosis in neighboring tissues.20,21 This combination of precision and minimal heat deposition makes it a suitable treatment for these patients with advanced metastases, enabling the creation of a cavity in the anterior portion of the vertebral body (instead of merely displacing tissue as seen in balloon-assisted kyphoplasty) for cement deposition and stabilization. A combined approach to treating these spinal metastases has been described in several publications,2,22 following the rationale that the tissue cavity allows more control over the injected cement and can help redirect flow away from a compromised posterior cortex or epidural extension and potentially decrease the extent of cement extravasation and posterior displacement of tumors into the spinal canal.
Cement augmentation was first proposed by Harrington23 in 1981 as a method to relieve pain associated with malignant spinal tumors even before the first vertebroplasty case was described. Harrington posited that spinal stabilization could be achieved by percutaneous cement augmentation, whereas decompression, if necessary, could be performed by posterior laminectomy and/or instrumentation, thus avoiding the anterior surgical approach.
A similar approach has also been described for the treatment of traumatic burst fractures,24,25 in which short-segment pedicle screw fixation combined with vertebroplasty or kyphoplasty is used in lieu of traditional long-segment fusion. Recently, Melcher et al26 presented a series of 40 cases in which combining balloon kyphoplasty with a posterior instrumentation and decompression in patients with pathologic fractures of the spine with involvement of the spinal canal led to a significant reduction in pain and disability. Spinal functionality was restored by reconstruction of vertebral body height and correction of angular deformity. Furthermore, neurologic complications could be avoided.
A close look at the results of studies in which the lesions are situated purely in the anterior portion of the vertebral body (15 levels) or the posterior portion of the vertebral body (13 levels) shows that attempts to create a targeted cavity area within the anterior part of the vertebral body indeed resulted in more control over cement deposition. This was most pronounced in posteriorly located lesions, where cement was deposited anterior to those lesions in 13 of 15 levels. In such lesions (zone IV Weinstein classification), standard vertebroplasty or kyphoplasty is contraindicated due to the potential of posterior cement leakages into the spinal canal. Coblation allows deposition of cement anterior to the lesion with proved stability and pain relief. The tumor lesion can eventually be treated by radiation therapy or chemotherapy depending on the type of tumor. On the basis of these findings, I recommend using this combined technique in those situations where using conventional vertebroplasty or kyphoplasty alone carries higher risks.
Pain Relief Associated with Cement Augmentation
The analgesic effects of cement augmentation in treating VCFs secondary to spinal metastases are well documented; a review of the literature by Halpin et al27 found that most studies reported significant pain reduction in 85%–97% of patients. Although the mechanism of pain relief in conventional vertebroplasty is unknown, with incomplete filling of the vertebral body offering significant pain relief,13,28 possible mechanisms may include stabilization of microfractures, redistribution of mechanical forces, and even the cytotoxicity of the PMMA in the cement, which could destroy nerve terminals.13,15,28,29
Although the follow-up period was short (2–4 weeks), 89% of our patients experienced pain relief. Long-term follow-up is extremely difficult in this patient population due to the high morbidity and mortality rates associated with these advanced cancers, making data collection difficult. However, a study of 37 patients with 1-year follow-up performed by Weill et al15 found that 94% of patients reported pain relief after 1 week, 73% at 6 months, and 65% at 1 year. Our comparable short-term results may indicate similar pain alleviation in the longer term. Pain relief immediately postprocedure has been projected as the best predictor of clinical outcome for the following 2 years after vertebroplasty.30
Cement Leakage
Although the reported complication rate for percutaneous vertebroplasty procedures is low, generally below 10%,31,32 cement leakage incidence as verified by CT can be as high as 81%.33 Despite the prevalence of cement leakage in these relatively safe procedures, effort must be made to minimize leakage because potentially severe pulmonary and neurologic complications can occur.34–39 In a retrospective study of 159 total percutaneous vertebroplasty procedures to treat 304 vertebral bodies, Barragán-Campos et al10 noted 423 incidences of cement leakage, though only 2 of these leakages resulted in systemic complications (pulmonary embolism resulting from cement migration through the vena cava). Postoperative radicular pain has also been theorized to stem from cortical leakage of cement into the foraminal space.40 Some of these studies were performed in the early days of vertebroplasty; the technique has seen considerable improvement during the last few years, with better delivery tools and thicker more radiopaque cement.
The advanced cases treated in this study were at greater risk for vertebroplasty complications; however, 30% of patients presented no leakage based on CT evaluation. All observed leakage, including 2 small epidural leaks, were minimal and clinically insignificant. Trumm et al41 reported a higher leakage rate than ours, based upon postprocedure CT examination of a series of 62 patients with breast cancer with spinal metastases who were treated with vertebroplasty. They reported intradiskal, intraspinal, and paravertebral leakage in 31%, 26%, and 26% of vertebrae, respectively.41
Cotten et al13 presented CT examinations of a series of 40 cases of malignant lesions treated with conventional vertebroplasty. This is the only published study to date that we are aware of concerning CT examination of cement leakage in vertebroplasty cases with malignant lesions. They reported leaks in 29 out of 40 cases studied (72.5%). Leaks were located in the spinal canal (n = 15), the neural foramina (n = 8), the adjacent disks (n = 8), the paravertebral tissue (n = 21), and the lumbar venous plexus (n = 2). Posterior cortical destruction and epidural extension were present in 13 and 10, respectively, of the 15 reported epidural leaks. Epidural leaks of methylmethacrylate were minimal, except in 2 patients, in whom marked posterior displacement of the thecal sac occurred without associated clinical neural compression.
Limitations of our study include its retrospective nature, the small number of patients, and the absence of a control group. However, this is a heretofore unexplored technique for the treatment and stabilization of advanced spinal metastases, and this retrospective evaluation is meant to serve as a foundation for future controlled prospective studies. Automated methodologies or software may serve as a superior method over direct visual observation for evaluating cement volumes. This study also did not elaborate on the effect of tumor-tissue debulking on the patient's prognosis. When removing bone and tumor material via coblation before augmentation in a cadaver model, Oakland et al42 noted a marginally significant improvement in relative failure strength following vertebroplasty.
Our study found that performing pmRF ablation before conventional vertebroplasty for treating advanced spinal metastases may result in more control over cement placement and the creation of a relatively predictable deposition pattern conforming to the anterior part of the vertebral body. This finding should be studied further, because the ability to generate a predictable deposition pattern may result in adoption of this treatment as a preferred course in the surgical management of many of these high-risk patients—especially those with zone III and IV lesions—to avoid an extensive anterior surgical approach. On the basis of this limited number of cases, this technique may be particularly useful in treating patients with malignant lesions located in the posterior part of the vertebral body, where conventional vertebroplasty and kyphoplasty are relatively contraindicated and carry higher complication rates. pmRF ablation may contribute to the safety of the procedure by decreasing the extent of cement leakage.
Footnotes
This work was supported by a grant from the ArthroCare Corporation.
Paper previously presented at: Annual Meeting of the American Society of Neuroradiology, May 31-June 6, 2008; New Orleans, La; and Annual Meeting of the American Society of Spine Radiology, February 20–24, 2008; Indian Wells, Calif.
indicates article with supplementary on-line table.
References
- Received November 19, 2008.
- Accepted after revision January 16, 2009.
- Copyright © American Society of Neuroradiology