Abstract
BACKGROUND AND PURPOSE: Neck musculature mass has been suggested as a biomechanical contributor to injury severity in mild traumatic brain injury. We sought to determine how the cross-sectional areas of the suboccipital muscles affect symptom severity, neurocognitive performance, and recovery time in patients with mild traumatic brain injury.
MATERIALS AND METHODS: Sixty-four consecutive patients with mild traumatic brain injury underwent MR imaging and serial neurocognitive testing with the Immediate Post-Concussion Assessment and Cognitive Test. Cross-sectional areas of the rectus capitis posterior musculature were retrospectively obtained at C1, and cross-sectional areas of the remaining 7 suboccipital muscles were measured at C2. Cross-sectional area reproducibility was evaluated. Overall and individual muscle cross-sectional areas were correlated with symptom severity, neuropsychological testing, recovery time, and headache.
RESULTS: Sixty-four patients with mild traumatic brain injury had imaging through C1, and 43 had imaging through C2. Reproducibility of cross-sectional area measurements was substantial (correlation coefficients = 0.9517–0.9891). Lower cross-sectional area of the rectus capitis posterior minor was correlated with greater symptom severity (r = 0.596, P < .0001), longer recovery time (r = 0.387, P = .002), poor verbal memory performance (r = 0.285, P = .02), and headache (r = 0.39, P = .001). None of the other cross-sectional areas were associated with symptom severity, recovery time, neurocognitive testing, or headache.
CONCLUSIONS: In mild traumatic brain injury, the rectus capitis posterior minor is the only suboccipital muscle whose cross-sectional area is associated with symptom severity and worse outcome. Given the unique connection of this muscle to the dura, this finding may suggest that pathology of the myodural bridge contributes to symptomatology and prognosis in mild traumatic brain injury.
ABBREVIATIONS:
- ΔV
- change in head velocity
- ImPACT
- Immediate Post-Concussion Assessment and Cognitive Test
- mTBI
- mild traumatic brain injury
- rectus capitis-PMaj
- rectus capitis posterior major
- rectus capitis-PMin
- rectus capitis posterior minor
Mild traumatic brain injury (mTBI), often referred to as “concussion,” is a common hazard in contact sports, with approximately 3.8 million sports-related injuries documented each year.1 Despite the outwardly mild nature of these injuries, approximately 15% of patients with mTBI have persistent, often debilitating symptoms beyond 3 months, termed “postconcussion syndrome.”2
The underlying injury in mTBI is theorized to be related to acceleration and deceleration of the brain within the cranial vault.3 Animal models have shown that the severity of brain injury is correlated with the change in head velocity (ΔV).4 Forces from a large ΔV predominantly impact frequent locations of shear injuries associated with postconcussive syndrome.5
In computer models of mTBI, early neck resistance is key in decreasing ΔV.6,7 As impact forces are proportional to ΔV,4,7 this means that very small reductions in ΔV by the neck musculature can result in a significant reduction in impact forces in regions associated with postconcussive syndrome.
Supporting the finding of increased neck strength and decreased ΔV, studies have shown that increased neck muscle strength results in decreased risk of postconcussion syndrome.8,9 However, increased overall neck strength has not resulted in alterations in ΔV during trauma in the experimental setting.10 This finding raises the question of whether specific muscles rather than overall strength are key to decreasing brain injury. Notably, muscles resisting head movement have been found central in determining outcome after linear acceleration injuries in whiplash.11
The suboccipital musculature is central to promoting and resisting head motion, including flexion, extension, and rotation.12 The rectus capitis posterior major (rectus capitis-PMaj), rectus capitis posterior minor (rectus capitis-PMin), semispinalis cervicis, multifidus, semispinal capitis, and splenius capitis are head extenders, while the longus colli and longus capitis are head flexors. The rectus capitis-PMaj, inferior oblique capitis, and semispinalis capitis are also involved in rotation. Because the cross-sectional area of muscles has previously been shown to be proportional to muscle strength,13,14 we sought to determine how the cross-sectional area of the suboccipital muscles affects symptom severity, neurocognitive performance, and recovery time in patients with mTBI.
Materials and Methods
Patient Selection and Image Acquisition
Our institutional review board approved this study with a waiver of informed consent. All MR imaging examinations were performed during the routine care of patients and were retrospectively reviewed.
We searched our electronic medical record to identify MR imaging studies performed for mTBI. Radiology reports from January 1, 2008, to July 31, 2013, were searched by using the keyword “concussion.” Inclusion criteria were 10–50 years of age, English language proficiency, and mild TBI defined as witnessed closed head trauma, no focal neurologic deficit, loss of consciousness of <1 minute, and posttraumatic amnesia of <30 minutes. Exclusion criteria were any abnormality on brain MR imaging as defined by a fellowship-trained neuroradiologist, including microhemorrhage/shear injury on gradient sequence (3 patients), the imaging not extending to C1 (4 patients), unavailable neurocognitive Total Symptom Score (4 patients), the Total Symptom Score being zero (3 patients), or excessive motion precluding accurate measurements (3 patients).
Neurocognitive testing was performed at the time of presentation, and the Immediate Post-Concussion Assessment Cognitive Test (ImPACT), a computerized test measuring cognitive function and postconcussion symptoms, was used. The ImPACT is the most scientifically validated and commonly used computerized neurocognitive evaluation system.15 It determines a total symptom score by using a 7-point Likert scale over 22 different categories and measures cognitive performance against normative data gathered on >17,000 athletes who participated in baseline testing as part of their pre-sport participation. The percentile rank for a subject's performance is determined by using the normative data from the control athletes of the same age group.16 After the initial neurocognitive testing, serial postconcussion symptom scores were obtained to determine the time to recovery, which was defined as the score being zero or the patient stating that he or she was asymptomatic.
Age and sex were recorded. Data collected included type of trauma, dates of injury and clinical evaluation, neurocognitive results, history of prior concussions, imaging results, clinical management, and any edema of the suboccipital musculature on T2 imaging. A prior concussion was defined as a diagnosis of concussion by an athletic trainer, neuropsychologist, or other medical personnel at any facility; however, documentation of that diagnosis had to be placed in the medical record. Recovery time was defined as when the patient stated that he or she was asymptomatic or the neurocognitive Total Symptom Score was zero.
MR imaging examinations were performed within 3 days of clinical examination on a 1.5T system (Signa; GE Healthcare, Milwaukee, Wisconsin) with a standard head coil and included axial images through C2. During the study period, all patients included in this study underwent the identical postconcussion imaging protocol on the same magnet system as follows: sequences included sagittal and axial T1-weighted imaging (TR, 600 ms; TE, minimum; section thickness, 5 mm; NEX, 1), and T2-weighted imaging (TR, 2000–2500 ms; TE, 84–102 ms; section thickness, 5 mm; NEX, 1). FOV ranged from 200 to 240 mm.
Suboccipital Muscle Cross-Sectional Area Calculations
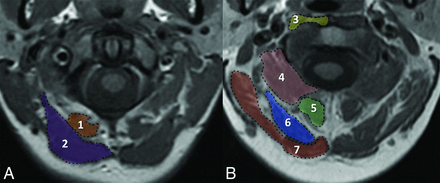
Neck muscle boundaries were manually outlined along their fascial borders by 2 radiologists on T1-weighted images with an orientation parallel to the foramen magnum with the assistance of a 3D viewer (Vitrea Core; Vital Images, Minnetonka, Minnesota). The cross-sectional area of the following muscles was evaluated at the C1 anterior arch: 1) rectus capitis-PMin, and 2) rectus capitis-PMaj; and the following, at the middens level: 3) longus colli and longus capitis (traced together), 4) inferior oblique capitis, 5) semispinalis cervicis and multifidus (traced together), 6) semispinalis capitis, and 7) splenius capitis musculature (Fig). Individual muscle cross-sectional areas were calculated, and total neck muscle cross-sectional area was determined by summing all of the individual neck musculature cross-sectional areas. Radiologists each reviewed 10 sample cases for training purposes. Reproducibility of muscle cross-sectional area measurements was then assessed on 20 test cases for each muscle group using 2 neuroradiologists, blinded to both the patient's history and the other observer's measurements. The Lin concordance correlation coefficient was used to evaluate agreement17 and was interpreted as follows18: poor agreement (<0.90), moderate agreement (0.90 to <0.95), substantial agreement (0.95–0.99), and almost perfect agreement (>0.99). Following the 20 test cases, a single neuroradiologist blinded to the patient's history made measurements.
Measurement of cross-sectional areas for the suboccipital muscles. Representative tracing of the cross-sectional areas on T1-weighted imaging of the rectus capitis posterior minor (1) and rectus capitis posterior major (2) muscles at the level of the anterior arch of C1 (A) and the longus colli/capitis (3), inferior oblique capitis (4), semispinalis cervicis/multifidus (5), semispinalis capitis (6), and splenius capitis (7) muscles at the middens level (B).
Univariate Data Analysis
Comparison of the demographic data was performed with a Fisher exact test or a 2-tailed t test. Comparison of cross-sectional area measurements was performed with an unpaired t test. Correlation of the cross-sectional area measurements with clinical metrics was performed with the Pearson correlation coefficient or a point-biserial coefficient. Correlation of clinical variables or muscle cross-sectional areas with recovery time was performed with a Pearson correlation coefficient or point-biserial coefficient. P values of < .05 were statistically significant.
Multivariate Data Analysis
Multivariate analysis for variables correlating with recovery time was performed with an ordinary least-squares model, by using variables whose P values were <0.10 by univariate analysis. Goodness of fit was evaluated with the Hosmer-Lemeshow statistic. Odds ratios and their 95% confidence intervals were calculated. P values < .05 were statistically significant.
Results
Patient Selection and Image Acquisition
Sixty-four patients were included (44 males, 20 females). A summary of the demographic and clinical data is shown in Table 1. No patients had macroscopic edema of the suboccipital musculature on T2-weighted imaging on the included FOV.
Clinical and demographic characteristics of patients with mTBI
Suboccipital Muscle Cross-Sectional Area Calculations
Reproducibility of the cross-sectional areas was substantial for all muscles (Lin correlation coefficients = 0.9517–0.9891) (Table 2). The average cross-sectional area measurements for the rectus capitis-PMin and rectus capitis-PMaj at C1 and the remaining suboccipital musculature at C2 are shown in Table 3.
Lin correlation coefficients for CSA of the muscles of head movement
Average CSAs of the suboccipital musculature
Univariate Data Analysis
The lower cross-sectional area of the rectus capitis-PMin was correlated with the following outcome measures: 1) greater symptom severity (r = 0.596; P < .0001), 2) longer recovery time (r = 0.387; P = .002), 3) poorer verbal memory performance (r = 0.285; P = .02), and 4) postconcussive headache (rpb = 0.39; P = .001). Neither the overall cross-sectional area nor those for any of the other individual muscles were associated with symptom severity, recovery time, neurocognitive testing, or headache. Among demographic factors, age and male sex correlated with recovery time on univariate analysis (r = 0.423 and −0.318; P = .005 and .01, respectively). Correlation results are summarized in the On-line Table.
Multivariate Analysis
Four variables had P values < .10 by univariate analysis: the rectus capitis-PMin cross-sectional area, longus coli/capitis cross-sectional area, age, and sex. Multivariate analysis found that the only statistically significant factor for prognosis was the rectus capitis-PMin cross-sectional area. A larger rectus capitis-PMin cross-sectional area was protective against a longer recovery time (adjusted odds ratio, 0.22; P = .03). Summary of the multivariate analysis is shown in Tables 4 and 5.
Variables trending towards correlation with recovery time (P <.10) on univariate analysis
Subsequent performance in a multivariate model
Discussion
In mTBI, a lower cross-sectional area of the rectus capitis-PMin alone among the suboccipital muscles was associated with greater symptom severity, longer recovery time, poor neurocognitive test performance, and postconcussive headache. Overall suboccipital muscle cross-sectional area did not correlate with clinical metrics or symptomatology after mTBI.
Suboccipital muscle atrophy has long been associated with chronic pain.19 Previous studies have shown greater atrophy in the rectus capitis-PMaj and rectus capitis-PMin among the suboccipital muscles in patients with persistent whiplash symptoms,20,21 and atrophy of these muscles has been associated with higher inflammatory biomarkers, hyperalgesia, and worse outcomes in patients with whiplash.22 However, these studies focusing on the effects of the suboccipital musculature on posttraumatic outcomes have focused exclusively on patients with whiplash-associated neck pain.23 No studies have extended these findings to patients with mTBI, who may not necessarily have an associated neck injury but often have an acceleration-deceleration energy transfer similar to that in whiplash injuries.24
Most interesting, decreased cross-sectional area in the rectus capitis-PMaj and rectus capitis-PMin musculature has also been found in patients with chronic tension-type headaches, in which the lower cross-sectional areas of the rectus capitis-PMaj and rectus capitis-PMin were associated with greater headache intensity, duration, and frequency.25 Tension-type headaches are among the most common headaches experienced after mTBI, with almost 40% of postconcussive headaches reported as tension headaches.26 However, the role of the rectus capitis-PMaj and rectus capitis-PMin in mTBI and their association with posttraumatic headaches have not been investigated, to our knowledge.
In our study, only the rectus capitis-PMin was associated with greater symptomatology, poorer outcome, and posttraumatic headaches after mTBI. Although the rectus capitis-PMaj and rectus capitis-PMin are both head extenders,20 the rectus capitis-PMin experiences the greatest load in low-energy impacts.27 In these low-energy injuries, the proportion of energy absorbed by the suboccipital muscles themselves is decreased relative to the strain on their tendons and connective tissue connections.27 The rectus capitis-PMin has a unique connective tissue bridge to the dura mater,28 which has been noted on both anatomic specimens and MR imaging.29⇓–31 This connective tissue bridge is responsible for resisting dural enfolding during neck extension. Traumatic injury to this myodural bridge can occur with a weak or atrophic rectus capitis-PMin. A smaller/weaker rectus capitis-PMin can absorb less energy, and as a result, higher energy is deposited in the myodural bridge, increasing the risk of injury.32 Secondary atrophy of the rectus capitis-PMin after trauma can also cause chronic dysfunction of the myodural bridge29 because an atrophic rectus capitis-PMin is less able to resist inward folding of the dura, resulting in abnormal dural movement and tension.33 This outcome can result in prominent referred pain because the dura itself is highly sensitive to tractional forces.
The dura is innervated by the first 3 cervical nerves, which converge with the trigeminal nerve in the trigeminal nucleus caudalis. Resulting activation of the nociceptors in the trigeminocervical nucleus by these cervical nerves produces a cervicogenic headache. It is therefore not surprising that the low cross-sectional area of the rectus capitis-PMin was associated with greater symptom severity and headaches in our cohort. In fact, cervicogenic headache from injury to the rectus capitis-PMin–dural connection is a well-known phenomenon in headaches from suboccipital procedures, where injury to the myodural bridge results in abnormal adhesions between the rectus capitis-PMin and the dura.34 Lysis of these abnormal rectus capitis-PMin–dural adhesions in these patients has been shown to provide symptom relief.35
Additional symptomatology associated with rectus capitis-PMin atrophy could arise from its role as the proprioceptive center of the upper cervical spine.36 The rectus capitis-PMin has the greatest concentration of muscle spindles among the suboccipital musculature,37 with an especially high concentration of large-diameter A-β fibers, which convey proprioceptive information. Transmission of proprioceptive data along these A-β fibers effectively blocks nociceptive signals from muscle C-fibers from reaching the spinal cord and higher order pain centers.36 Atrophy of the rectus capitis-PMin results in a decrease in A-β fibers, which, in turn, causes less inhibitory signals and greater pain impulses to central pain pathways.
Cognitive difficulties are commonly seen in patients with both acute and chronic pain,38 and pain is one of the most significant contributors to neurocognitive performance after mTBI.39 Thus, rectus capitis-PMin atrophy may play a role in both the symptomatology and cognitive deficits after mTBI. Together, these findings may indicate a role for preventive strengthening exercises focused on the rectus capitis-PMin musculature in individuals at high-risk for mTBI.
Our study has limitations. Our evaluation was a retrospective, single-institution study with a moderate sample size. Accordingly, the findings should be corroborated with a larger prospective study. Furthermore, our study included both patients who were thought to warrant imaging clinically and those with prior concussions. Thus, a selection bias may exist toward more seriously injured patients who present with significant symptoms that warrant imaging. Arguably, although a bias exists, it is a bias toward the patients that would most benefit from imaging biomarkers. Additionally, only the suboccipital muscles were evaluated in our study, and further studies evaluating the relationship of the lower neck muscles to symptoms and outcomes in mTBI would help to better understand how the biomechanical and physiologic properties of the neck affect what has often been considered exclusively brain pathology.
Conclusions
In mild TBI, the rectus capitis-PMin is the only suboccipital muscle whose cross-sectional area is correlated with symptom severity and worse outcome. This may reflect greater strain on the myodural bridge in patients with a smaller rectus capitis-PMin or perhaps decreased inhibition of nociceptive pathways from rectus capitis-PMin spindle atrophy. Understanding how suboccipital muscle loss influences the pathophysiology of mTBI may help develop physical therapy rehabilitation programs to improve outcomes in this population.
References
- Received September 29, 2015.
- Accepted after revision January 13, 2016.
- © 2016 by American Journal of Neuroradiology