Abstract
BACKGROUND AND PURPOSE: Perfusion CT may improve the diagnostic performance of noncontrast CT in acute ischemic stroke. We assessed predictors of focal hypoperfusion in acute ischemic stroke and perfusion CT performance in predicting infarction on follow-up imaging.
MATERIALS AND METHODS: Patients from the Acute STroke Registry and Analysis of Lausanne data base with acute ischemic stroke and perfusion CT were included. Clinical and radiologic data were collected. We identified predictors of focal hypoperfusion using multivariate analyses.
RESULTS: From the 2216 patients with perfusion CT, 38.2% had an acute ischemic lesion on NCCT and 73.3% had focal hypoperfusion on perfusion CT. After we analyzed 104 covariates, high-admission NIHSS, visual field defect, aphasia, hemineglect, sensory deficits, and impaired consciousness were positively associated with focal hypoperfusion. Negative associations were pure posterior circulation, lacunar strokes, and anticoagulation. After integrating radiologic variables into the multivariate analyses, we found that visual field defect, sensory deficits, hemineglect, early ischemic changes on NCCT, anterior circulation, cardioembolic etiology, and arterial occlusion were positively associated with focal hypoperfusion, whereas increasing onset-to-CT delay, chronic vascular lesions, and lacunar etiology showed negative association. Sensitivity, specificity, and positive and negative predictive values of focal hypoperfusion on perfusion CT for infarct detection on follow-up MR imaging were 66.5%, 79.4%, 96.2%, and 22.8%, respectively, with an overall accuracy of 76.8%.
CONCLUSIONS: Compared with NCCT, perfusion CT doubles the sensitivity in detecting acute ischemic stroke. Focal hypoperfusion is independently predicted by stroke severity, cortical clinical deficits, nonlacunar supratentorial strokes, and shorter onset-to-imaging delays. A high proportion of patients with focal hypoperfusion developed infarction on subsequent imaging, as did some patients without focal hypoperfusion, indicating the complementarity of perfusion CT and MR imaging in acute ischemic stroke.
ABBREVIATIONS:
- AIS
- acute ischemic stroke
- ASTRAL
- Acute STroke Registry and Analysis of Lausanne
- FHP
- focal hypoperfusion
- MVA
- multivariate analysis
- PCT
- perfusion CT
Neuroimaging plays a major role in the evaluation of patients with acute ischemic stroke (AIS). CT-based imaging is currently the most frequently used acute technique, differentiating ischemic from hemorrhagic stroke and identifying some stroke mimics.1,2 It is also the most commonly used method for selecting patients for endovascular recanalization treatment3 and has shown promise in predicting treatment response4 and improving clinical outcome.5 However, its limited sensitivity for detecting early ischemia is a major drawback, even if scores of systematic analysis of CT may improve patient selection.6,7 Thresholds in perfusion imaging are still subject to debate,8 despite progress in the field,9 however.
Knowledge of the performance of perfusion CT (PCT) in predicting focal hypoperfusion (FHP) in AIS may help to improve stroke recognition10⇓⇓⇓–14 and thereby decrease the proportion of stroke mimics. Better diagnosis of stroke mimics and “chameleons”15 will lead to better patient disposition and lower thrombolysis rates of mimics.16
The aims of this study were to determine the clinical and radiologic predictors of FHP on PCT in AIS and to assess the diagnostic performance of FHP for infarction on follow-up imaging.
Materials and Methods
Study Population
All consecutive patients admitted to the stroke unit and/or intensive care unit of the Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, with a main diagnosis of AIS were included in the Acute STroke Registry and Analysis of Lausanne (ASTRAL), from January 1, 200317 to June 30, 2015. AIS was diagnosed according to the World Health Organization definition.18 Only patients arriving in our emergency department within 24 hours of AIS onset or at last proof of good health were included. Patients with transient ischemic attack, intracerebral hemorrhage, subarachnoid hemorrhage, and cerebral sinus venous thrombosis were collected in another register.
The indication for PCT imaging in our institution is suspected AIS with the patient arriving within 24 hours of onset; for pure clinical posterior fossa syndrome, the decision to perform PCT is left to the treating physician.17 Patients with known iodinated contrast allergy, known renal clearance of <30 mL/minute, and severe agitation do not undergo PCT.
For the present analysis, we selected all patients in the ASTRAL with a good-quality PCT performed within 24 hours of onset. PCT was considered of good quality if the arterial input curve re-descended to baseline before the end of image acquisition and if the rise of the venous transit curve occurred later than the arterial one and if there were no major movement artifacts and at least 2 good-quality slices available for analysis. Patients with NCCT only or MR imaging in the acute phase were excluded. A total of 104 variables regarding demographics, vascular risk factors, clinical information, acute laboratory findings, stroke localization, acute neuroimaging findings, and acute treatment were used for analysis and are listed in Table 1 and On-line Tables 1 and 2.
Selected patient characteristics from the overall patient population and in patients with and without FHPa
Cerebral CT Imaging Protocol
Cerebral CT was performed on a 16–detector row multidetector CT scanner (LightSpeed; GE Healthcare, Milwaukee, Wisconsin) until November 2005 and on a 64–detector row multidetector CT scanner (LightSpeed VCT; GE Healthcare) thereafter. NCCT, PCT, CT angiography, and postcontrast series were acquired.
NCCT and postcontrast series were acquired in the axial mode from the skull base to the vertex (16-cm z-axis coverage) using the following imaging parameters: 120-kV(peak) tube voltage, 320-mA tube current, slice thickness = 5 mm, 32-cm scan FOV, 512 × 512 matrix.
All PCT series were acquired in the axial scan mode with 80-kVp tube voltage, 120-mA tube current, 32-cm scan FOV, and 512 × 512 matrix, as described in Wintermark et al.19 PCT was positioned at the level of the basal ganglia and the third ventricle above the orbits to protect the lens. Eighteen groups of 4 slices of 10 mm (40-mm z-axis coverage) were used until November 2005; then, 18 groups of 16 slices of 5 mm (80-mm z-axis coverage) were used from November 2005 to June 2015. Hence, the PCT series did not include the posterior fossa in the first 3 years of the study; thereafter, the PCT series usually included the upper half of the posterior fossa due to the increased z-axis coverage of the 64–detector row multidetector CT scanner. PCT images were acquired during 50 seconds in a cine mode with a delay of 5–7 seconds after injection of 50 mL of iodinated contrast (Accupaque 300, iohexol 300 mg/mL; GE Healthcare) at a flow rate of 5 mL per second, followed by 50 mL of 0.9% saline solution at the same flow rate into an antecubital vein using a power injector.
PCT data were re-analyzed off-line specifically for research purposes using the most recent Brilliance Workspace Portal (Philips Healthcare, Best, the Netherlands) and on the basis of the central volume principle using deconvolution to create parametric maps of mean transit time. Cerebral blood volume was calculated from the area under the time-enhancement curves, and cerebral blood flow was derived from the formula CBF = CBV / MTT. Regarding deconvolution, we always used an appropriate threshold for MTT, TTP, CBV, and CBF, as described by Man et al.20
CT angiography was acquired in the helical scan mode (parameters: 120-kVp tube voltage, 150- to 260-mA tube current, 0.984 pitch, 0.625-mm slice thickness, 50-cm scan FOV, 512 × 512 matrix) from the aortic arch to the top of the frontal sinuses after injection of 50 mL of iodinated contrast (Accupaque 300, 300 mg/mL) at a flow rate of 5 mL per second (same parameters as for perfusion data) followed by 50 mL of 0.9% saline solution at the same flow rate.
Cerebral CT Imaging Analysis
An experienced vascular neurologist (P.M.) and 2 senior neuroradiologists, all with 15-years' experience reading multimodal CT, independently reviewed NCCT, PCT, MR imaging, and CTA images. In cases of discordance, a consensus was found in multidisciplinary meetings, with awareness of the type and side of the clinical deficits. On NCCT, early ischemic changes, ASPECTS, the hyperdense middle cerebral artery sign, chronic or subacute infarct, and leukoaraiosis were recorded. On PCT, FHP was defined as clearly prolonged MTT visible on >1 slice, corresponding to an arterial territory that was not attributable to an underlying chronic tissue lesion and consistent with the clinical syndrome. Hence, we used any discernable visual change in the MTT parameter maps as a sign of FHP rather than a numeric threshold.
We chose MTT because it is a more sensitive marker of focal hypoperfusion than CBF21⇓–23 (with a small risk of overdiagnosing ischemia however) and it has high interrater agreement in our institution (κ = 0.79 in 100 patients with acute supratentorial stroke symptoms) and in the literature.24,25
The raw axial and maximal-intensity-projection CTA images were also reviewed for significant (≥50% stenosis or occlusion) extra- and intracranial arterial pathology leading to the ischemic territory.
Follow-up imaging (either CT or MR imaging) performed any time after 24 hours of onset was reviewed for new ischemic lesions corresponding to the clinical picture.
Statistical Analysis
All analyses were conducted using R-3.2.3 (http://www.r-project.org/). Continuous variables are shown as median (interquartile range). First, a univariate comparison of patients with and without FHP was performed for all acute-phase variables (Table 1 and On-line Tables 1 and 2). Then, a multivariate analysis (MVA) was performed using all variables available before neuroimaging (ie, demographic and clinical data and AIS characteristics such as localization, stroke mechanism) to assess the association of these variables with FHP before neuroimaging. Finally, a second MVA was conducted in which all pertinent radiologic data (early ischemic changes, leukoaraiosis, chronic infarcts, significant arterial pathology) were added to the first MVA, to include all available information pertinent for hyperacute-treatment decision-making. In all multivariate analyses, imputation of missing data was performed using the chained equations methodology. Five imputed datasets were generated for each MVA. We performed a separate analysis on each imputed dataset, determining the important covariates associated with the response using backward elimination methods. The finalized outputs of the imputed analyses were appropriately combined to produce the results of this study.
Bivariate associations between each predictor and the outcomes were assessed using a logistic regression model. The odds ratio, 95% confidence intervals, and associated P values quantified the strength of the association between the predictors and the response in both univariate and multivariate logistic analyses. Significance was set at the .05 (5%) level.
Results
A total of 3322 AISs were registered in ASTRAL during the 12-year observation period. Of these, 1031 (31.0%) patients were excluded because they did not undergo PCT on admission. Reasons for PCT exclusions are shown in On-line Figure: acute MR imaging performed instead of CT (n = 45), known/suspected iodine contrast allergy (n = 46), not attempted because the patient was agitated or moving (n = 9), PCT performed later than 24 hours after AIS onset (n = 70), renal insufficiency (n = 204), AIS considered as vertebrobasilar (n = 317), and physician's choice (n = 340). After we excluded 75 others (2%) for technical reasons, (<2 PCT slices available for analysis [n = 4], injection failure [n = 32], or movement artifacts [n = 39]), 2216 patients remained in the analysis, of which 467 (21%) were admitted between November 2003 and 2005, and 1749 (79%), between December 2005 and June 2015.
Excluded patients had similar age and sex, higher preadmission mRS scores, less severe admission NIHSS scores, and more frequent cardioembolic and lacunar stroke etiology in the univariate comparison (On-line Tables 1 and 2).
The demographics, risk factors, and clinical data of the included population are listed in Table 1 and On-line Tables 1 and 2. Overall, 831 (38.2%) had early ischemic changes on NCCT, and 1624 (73.3%) had FHP on PCT. In 532 (24%), findings of both NCCT and PCT were considered normal. For the 1404 patients admitted within 6 hours of AIS onset, 506 (36%) had early ischemic changes on NCCT and 1027 (73.2%) had FHP on PCT.
Among the 1657 patients with pure anterior circulation AIS, NCCT showed early ischemic changes in 710 (43.1%) and FHP was seen on PCT in 1393 patients (86.6%). For the 394 with pure posterior circulation AIS, the numbers were 75 (19%) and 182 (11.3%), respectively.
Considering preimaging data in the MVA, acute NIHSS, the presence of cortical signs (hemineglect, aphasia, and visual field defects), sensory deficits, and impaired consciousness all showed significant association with FHP. Pretreatment with anticoagulants, pure posterior circulation localization, and lacunar etiology were less associated with FHP (Table 2).
Multivariate analysis of clinical and combined clinical and radiologic variables associated with FHP
In the second MVA with added acute neuroimaging findings, FHP was independently associated with hemineglect, visual field defects, sensory deficits, early ischemic changes on NCCT, anterior circulation localization, significant arterial pathology in the ischemic territory, and cardiac stroke mechanism. Increasing onset-to-CT delay, chronic vascular lesions, and lacunar mechanism were negatively associated with FHP (Table 2).
This study also investigated the performance of NCCT and acute PCT findings regarding predicting a definitive infarct lesion on follow-up imaging in the subacute or chronic phase. From the 2216 patients, 873 patients had at least 1 follow-up MR imaging, 980 had a subsequent CT, and 363 patients had no follow-up imaging.
On the basis of these patient data, we calculated the sensitivity and specificity of admission NCCT to predict a follow-up lesion on any type of imaging as 43.3% and 96.6%, respectively (Table 3).
Accuracy of PCT as a diagnostic test for infarct identification on follow-up CT and MRI
In the same population, the sensitivity of FHP to detect a lesion on any follow-up imaging was 80.1%, and 66.5% if only MR imaging follow-up images were considered, indicating that a substantial number of FHPs do not necessarily turn into visible infarction.
The specificity of FHP on acute PCT (for the absence of a lesion on any follow-up imaging) was 57.9%, and 79.4% if only MR imaging follow-up images were considered. This finding indicates that about 20% of PCTs show that patients have FHP, without a visible lesion on later MR imaging (Fig. 1).
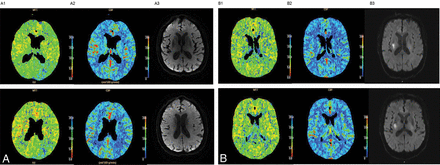
A, Patient with left MCA stroke, NIHSS 4, and acute PCT showing an FHP corresponding to the clinical deficit (A1, MTT and A2, CBF). Subacute MR imaging at 3 days shows no DWI lesion (A3). B, A patient with right MCA stroke, NIHSS 6, and normal findings on acute PCT (B1, MTT and B2, CBF), but an acute DWI lesion at 1 day after admission (B3) corresponding to the clinical deficit. These examples convey the complementarity of acute PCT and MR imaging.
The positive predictive value of FHP toward an infarct was 91.2% (96.2% considering only MR imaging lesions), indicating that PCT has very few false-positives (Table 3).
The diagnostic accuracy for infarct detection on follow-up imaging was 76.8% for PCT and 51.2% for NCCT.
Discussion
In this comprehensive analysis of a large number of consecutive AISs, we show that FHP is associated with cortical semiology, anterior circulation ischemia, nonlacunar strokes, earlier imaging, and the presence of arterial pathology and FHP has a moderate sensitivity, high specificity, and very high positive predictive value for radiologically visualized lesion development on MR imaging. Overall, PCT doubles the sensitivity of AIS detection compared with NCCT.
Clinical and Radiologic Predictors of FHP on PCT
Among the 8 clinical variables independently predicting FHP (Table 2), NIHSS, hemineglect, aphasia, and visual field defects on admission were expected because they are frequently associated with hemispheric lesions of large volume; furthermore, the ischemic lesions associated with these variables tend to be covered by PCT protocols.26 The posterior fossa, however, is covered incompletely by most current PCT acquisitions, and sensitivity in this region might be further lowered by the smaller lesion volumes and radiation absorption by bone and bone artifacts.11,27 The association between FHP and decreased level of consciousness is likely explained by the large hemispheric lesions that have a high detection rate on PCT. AIS of lacunar origin showed FHP in only 2.7% of cases, probably because of the limited resolution of PCT.28 The finding that anticoagulation was negatively associated with FHP in the MVA may be explained by the tendency of anticoagulation to protect against large emboli.29,30 Following the clinical associations, we found a patient presenting with a rather severe hemispheric syndrome and normal PCT findings should be suspected of having an AIS mimic. Similarly, a clinician who wants to confirm AIS in a patient with minor symptoms of lacunar origin or with a posterior fossa syndrome should directly perform MR imaging as the initial study.
When we added imaging data to the MVA (Table 2), the finding that a shorter delay from symptom onset to CT was a predictor of FHP is likely explained by patients with more severe strokes arriving more rapidly to the hospital and therefore imaging,31 as well as the increasing probability of spontaneous recanalization with time.32⇓–34 Not surprising, abnormal arterial findings (stenosis of >50% or occlusion) were also associated with FHP on PCT, which may help the radiologist identify stenosis or occlusion in distal arteries. In contrast to other studies,26,35 we did not find an association between the volume of brain covered by PCT (number of slices) and FHP.
Diagnostic Performance of FHP for Infarction on Follow-Up Imaging
The performance of PCT for the prediction of a subsequent infarct on any type of follow-up imaging was 80.1% sensitive, and 66.5% if only MR imaging was used for follow-up, compared with 43.3% sensitivity for NCCT. The low rate of early ischemic changes on NCCT could be explained by the rapid onset-to-hospital timings (median, 3 hours). The almost doubled detection rate of AIS by PCT compared with NCCT is very welcome, particularly if diagnosis of AIS is uncertain and when DWI-based MR imaging is not available.7 We would therefore recommend adding PCT, especially in patients with cortical symptoms.
The specificity of PCT using MR imaging as a reference standard was high (79.4%), indicating that in 20% of patients with findings negative for infarct on MR imaging, FHP was visible by acute PCT. Nevertheless, acute DWI remains more sensitive than multimodal CT–based imaging, as described in previous studies.7 However, our findings speak for the complementarity of both techniques to diagnose AIS.
Strengths and Limitations
Strengths of this analysis include the large number of consecutive PCTs during a long period and the use of prespecified data using up-to-date scales, definitions, and neurovascular imaging methods. The large database allowed us to test multiple clinical and radiologic variables, increasing the chances of obtaining significant results in multivariate analyses. Limitations of our work include being a single center and the retrospective, observational, noncontrolled, and nonrandomized nature of the study, without previous power calculation. In addition, we excluded patients without PCT imaging or with data of insufficient quality. However, the excluded patients were quite similar to the studied population except for the lower NIHSS scores and more cardioembolic strokes. Our study design did not allow testing the accuracy of PCT to diagnose AIS, and indeed, limitations of PCT include only showing selected brain areas, with the risk of some ischemic areas not being covered. In addition, our PCT methodology evolved during the observation period: The number of slices increased and slice thickness decreased. Brain coverage with PCT was less complete before November 2005, particularly for the vertex and posterior fossa. Most interesting, in our study, the number of slices available for analysis did not influence the FHP detection rate, and modification of the reconstruction algorithm did not seem to influence PCT parameters at a regular radiation dose.36
We used visually clear prolonged MTT in a focal vascular area to detect FHP rather than a numeric threshold that is more objective. Thresholds for ischemia in perfusion imaging remain a matter of debate,8 however, and may not add to the clinical question of ischemia detection, which is why we looked at FHP in the context of a clinical syndrome. Another drawback of using MTT could be overdiagnosis of ischemia in cases of benign oligemia25 distal to an arterial stenosis. An alternative method to PCT for detection of hypoperfusion is polyphasic CTA, which adequately selected patients in a large randomized clinical trial37 and with less radiation exposure and shorter processing time. Finally, the follow-up imaging included all imaging performed in the subacute or chronic phase, which could lead to heterogeneous results but is consistent with common practice.
Conclusions
The present work identifies independent predictors of FHP on PCT in AIS, potentially allowing detection of more stroke mimics (from normal PCT findings) and more chameleons (from unexpected FHP in an acute neurologic deficit). It also indicates that acute PCT may add to the AIS diagnostic value in conjunction with NCCT and delayed MR imaging. Taken together, these could potentially improve AIS management.
Acknowledgments
We acknowledge Melanie Price Hirt for English language correction and editing and Ashraf Eskandari for data collection and extraction.
Footnotes
Disclosures: Max Wintermark—UNRELATED: Consultancy: More Health, Magnetic Incisght, icometrix, SUBTLE, Nines. Patrik Michel—RELATED: Grant: Swiss National Science Foundation, Swiss Heart Foundation; UNRELATED: Grant: Bristol-Myers Squibb, Comments: research grant, advisory board; Board Membership: Pfizer, Comments: advisory board; Consultancy: Medtronic, Amgen, Comments: consulting fees, speakers fees from Medtronic; OTHER RELATIONSHIPS: serves/recently served on the steering committee of BASICS, ELAN, the International PFO-Consortium, SOCRATES, and PROMISE, and the DSMB of CLOSE. All these remunerations and fees are used for stroke education and research.
References
- Received September 21, 2018.
- Accepted after revision December 30, 2018.
- © 2019 by American Journal of Neuroradiology