Abstract
BACKGROUND: The mean ADC value of the lower Gaussian curve (ADCL) derived from the bi-Gaussian curve-fitting histogram analysis has been reported as a predictive/prognostic imaging biomarker in patients with recurrent glioblastoma treated with bevacizumab; however, its systematic summary has been lacking.
PURPOSE: We applied a systematic review and meta-analysis to investigate the predictive/prognostic performance of ADCL in patients with recurrent glioblastoma treated with bevacizumab.
DATA SOURCES: We performed a literature search using PubMed, Scopus, and EMBASE.
STUDY SELECTION: A total of 1344 abstracts were screened, of which 83 articles were considered potentially relevant. Data were finally extracted from 6 studies including 578 patients.
DATA ANALYSIS: Forest plots were generated to illustrate the hazard ratios of overall survival and progression-free survival. The heterogeneity across the studies was assessed using the Cochrane Q test and I2 values.
DATA SYNTHESIS: The pooled hazard ratios for overall survival and progression-free survival in patients with an ADCL lower than the cutoff values were 1.89 (95% CI, 1.53–2.31) and 1.98 (95% CI, 1.54–2.55) with low heterogeneity among the studies. Subgroup analysis of the bevacizumab-free cohort showed a pooled hazard ratio for overall survival of 1.20 (95% CI, 1.08–1.34) with low heterogeneity.
LIMITATIONS: The conclusions are limited by the difference in the definition of recurrence among the included studies.
CONCLUSIONS: This systematic review with meta-analysis supports the prognostic value of ADCL in patients with recurrent glioblastoma treated with bevacizumab, with a low ADCL demonstrating decreased overall survival and progression-free survival. On the other hand, the predictive role of ADCL for bevacizumab treatment was not confirmed.
ABBREVIATIONS:
- ADCL
- mean ADC value of the lower Gaussian curve
- HR
- hazard ratio
- OS
- overall survival
- PFS
- progression-free survival
- QUADAS-2
- Quality Assessment of Diagnostic Accuracy Studies 2
- VEGF
- vascular endothelial growth factor
Glioblastoma remains the most common and lethal primary malignant tumor of the CNS, with a median overall survival of 8–14 months despite aggressive surgery, chemotherapy, and radiation.1,2 Histologically, glioblastoma is characterized by tumor cell anaplasia, necrosis, and prominent angiogenesis mediated by the vascular endothelial growth factor (VEGF), constituting a rationale for targeted therapy.3 Bevacizumab is a humanized anti-VEGF monoclonal immunoglobulin 1 antibody, and its use for recurrent glioblastoma was approved by the US Food and Drug Administration in 2009. Although several clinical trials failed to demonstrate its contribution to extending patient survival in newly diagnosed glioblastoma or progressive glioblastoma,4⇓-6 prolonged progression-free survival (PFS) and overall survival (OS) have been reported in the recurrence setting with either bevacizumab alone or in combination with other chemotherapy.7
MR imaging is an essential imaging technique for diagnosis, treatment planning, and evaluation of therapeutic effects in patients with glioblastoma. A meta-analysis by Choi et al8 demonstrated the benefit of perfusion MR imaging, including dynamic susceptibility contrast MR imaging and dynamic contrast-enhanced MR imaging, as a predictive and prognostic imaging biomarker in patients with recurrent glioblastoma treated with bevacizumab. There have also been many studies investigating the performance of DWI as a predictive/prognostic imaging biomarker in recurrent glioblastoma.9⇓⇓⇓⇓⇓⇓⇓⇓-18 In particular, several studies have reported the usefulness of the mean ADC value of the lower Gaussian curve (ADCL) derived from the bi-Gaussian curve-fitting histogram analysis;9⇓⇓⇓⇓-14 however, a systematic summary of this topic has been lacking.
The purpose of this study was to systematically review the literature and investigate the predictive/prognostic role of pretreatment DWI, especially ADCL, in patients with recurrent glioblastoma receiving bevacizumab treatment.
MATERIALS AND METHODS
Protocol
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 statement.19
Study Selection
We searched the PubMed, Scopus, and EMBASE data bases using the following search terms on August 24, 2021, without any language or date limits:
((glioblastoma)OR(GBM)) AND ((avastin) OR (bevacizumab)) AND ((ADC) OR (apparent diffusion coefficient)) for PubMed
(glioblastoma OR gbm) AND (avastin OR bevacizumab) AND (adc OR (apparent AND diffusion AND coefficient)) for EMBASE
ALL ((glioblastoma OR gbm) AND (avastin OR bevacizumab) AND (adc OR (apparent AND diffusion AND coefficient) AND (LIMIT-TO (SUBJAREA, “MEDI”)) for Scopus
Publications that met all of the following criteria were considered eligible:
Participants: patients were clinically, radiologically, and/or histologically diagnosed with recurrent glioblastoma treated with bevacizumab and underwent pretreatment MRI
Index test: ADCL
Outcome: hazard ratio (HR) for PFS or OS between patients with high and low ADCL
Study design: retrospective or prospective observational studies and clinical trials.
Exclusion criteria were as follows:
The full text was unavailable.
It was not a peer-reviewed journal publication.
The relationship between ADC and survival was not analyzed, or the HRs were not calculated or could not be estimated.
The index was not ADCL.
Possible duplication of patients: the study with a smaller number of patients was excluded.
Review, systematic review, or meta-analysis.
Non-English references were translated into English using Google Translate (translate.google.com) and examined.
Data Analyses
Two board-certified radiologists with 9 and 6 years of experience in neuroradiology reviewed the full text of the eligible studies by consensus. Any disagreements were resolved by another board-certified radiologist with 13 years of experience in neuroradiology. We collected authors names, publication year, the region where patients were included, period of patient inclusion, study methods, trial name, number of patients, mean or median age, sex, the definition of recurrence of glioblastoma, treatment regimen, MR imaging vendor/model/magnetic field strength, b-values, ROI placement method, ADC type, the cutoff value of ADC, how the cutoff value was determined, and outcomes (HRs for OS and PFS). When the original study did not report the 95% confidence intervals of HRs, we estimated them using all the available data from the reported statistics.
Quality and Risk-of-Bias Assessment
We used the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2).20 QUADAS-2 is based on the 4 domains (patient selection, index test, reference standard, and flow and timing). These domains are assessed regarding the risk of bias, and the first 3 domains are also assessed in terms of applicability.
Statistics
A forest plot was generated to illustrate the HRs for OS and PFS in patients with an ADCL lower than the cutoff values along with upper and lower 95% CIs. The pooled HR with 95% CIs was calculated using the fixed-effects model. The heterogeneity across the studies was assessed using the Cochrane Q test and I2 values. I2 values were interpreted as follows: 0%–29%, low; 30%–49%, moderate; and 50%–90%, considerable heterogeneity. P values < .05 were considered statistically significant. Statistical analyses were performed using Review Manual (RevMan, Version 5.4; https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman).
RESULTS
Study Selection
A total of 1344 abstracts were screened, of which 113 duplications were excluded. A total of 1148 articles were excluded by title and abstract screening. After excluding 77 articles according to the inclusion/exclusion criteria, finally, 6 studies including a total of 578 patients met the selection criteria for the systematic review.9⇓⇓⇓-14 The process of study selection is summarized in Fig 1. Publications in this systematic review ranged from 2012 to 2020.
Flow diagram of study selection.
Study Characteristics
The individual study characteristics are summarized in the Online Supplemental Data. A total of 578 patients with recurrent glioblastoma treated with bevacizumab and 236 patients treated without bevacizumab (hereafter, the bevacizumab-free cohort) were included. The mean or median patient age was approximately 50 years in each study. The VOI was acquired in the enhancing tumor areas, and ADC histogram analysis was performed with the bi-Gaussian fitting model, though 1 study used the 3 peaks model.10 The ADCL was generated on the basis of the fitting curve, and OS and PFS were compared using cutoff values of 1.050–1.240 × 10−3mm2/s and 1.050–1.241 × 10−3mm2/s, respectively. The cutoff value was determined by averaging,9,13,17 the hierarchical Bayesian method,14 where the OS difference among patient cohorts was the largest,12 or based on empiric thresholds identified in previous studies,11 though the method was not described in detail in 1 study.10 These studies excluded the areas of macroscopic cystic, hemorrhagic, and necrotic changes from the VOIs for ADC histogram analyses. Three studies11,12,14 compared the OS of the bevacizumab-free cohort depending on the ADCL, though only 2 studies compared PFS.11,14
Quality and Risk-of-Bias Assessment
The results of QUADAS-2 are summarized in the Online Supplemental Data. Most studies had a low risk of bias in terms of patient selection, index test, reference standard, and flow and timing. Studies that did not describe whether the participants were consecutive, randomized, or neither were considered to have an uncertain risk of bias regarding the patient selection. A study in which the method of the definition of ADCL and the cutoff value determination were not described in detail was considered to have an uncertain risk of bias regarding the index test and reference standard.
Meta-analysis
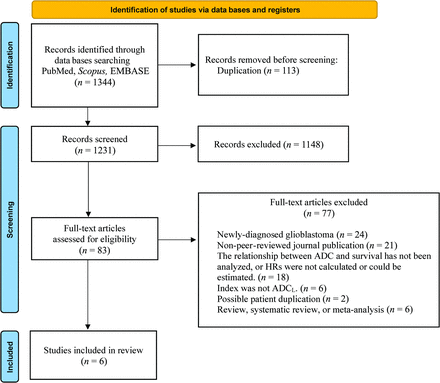
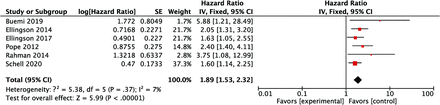
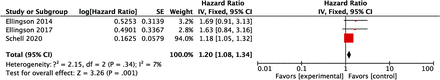
HRs for OS in patients with an ADCL lower than the cutoff values were available in all of the 6 studies, and the pooled HR of the lower ADCL was 1.89 (95% CI, 1.53–2.32), indicating worse survival (Fig 2). The lower 95% CI of HRs for OS was >1.00 in all studies. The heterogeneity of HRs for OS was considered low with the Q value in the Cochran Q test of 5.38 (P = .37) and an I2 of 7%. The comparison of HRs for PFS was available in 5 studies, and the pooled HR with an ADCL lower than the cutoff values was 1.98 (95% CI, 1.54–2.55) (Fig 3). Although the lower 95% CI of HR for PFS was <1.00 in 1 study,10 the heterogeneity of HRs for PFS was low, with the Q value in the Cochran Q test of 5.63 (P = .23) and an I2 of 29%. For studies with a bevacizumab-free cohort, no differences in OS or PFS were found, depending on the ADCL in 2 of 3 studies,11,12 whereas the significant differences in OS and PFS were retained in the bevacizumab-free cohort in 1 study.14 The pooled HR of the lower ADCL for OS in this subgroup was 1.20 (95% CI, 1.08–1.34) with a low heterogeneity (the Q value in Cochran Q test was 2.15 [P = .34] and the I2 was 7%; Fig 4).
A forest plot summarizing the HR of OS in patients treated with bevacizumab with the ADCL lower than the cutoff values compared with those with the ADCL higher than the cutoff values. SE indicates standard error; IV, instrumental variable.
A forest plot summarizing the HR of PFS in patients treated with bevacizumab with the ADCL lower than the cutoff values compared with those with the ADCL higher than the cutoff values. SE indicates standard error; IV, instrumental variable.
A forest plot summarizing the HR of OS in the bevacizumab-free cohort with the ADCL lower than the cutoff values compared with those with the ADCL higher than the cutoff values. SE indicates standard error; IV, instrumental variable.
DISCUSSION
DWI is a unique technique that allows noninvasive observation of the microstructure of tumors and surrounding brain tissues and is widely used in daily clinical practice. In this study, the high prognostic performance of the ADCL for survival was confirmed in patients with recurrent glioblastoma treated with bevacizumab. The meta-analysis demonstrated that a lower ADCL on pretreatment MR imaging was related to unfavorable survival with pooled HRs of 1.89 (95% CI, 1.53–2.32) for OS and 1.98 (95% CI, 1.54–2.55) for PFS, with low heterogeneity among the studies. However, the subgroup analysis with the patients treated without bevacizumab (the bevacizumab-free cohort) also showed a high pooled HR for OS of 1.20 (95% CI, 1.08–1.34), indicating that although the ADCL has a prognostic value, uncertainty remains as to whether the ADCL has a predictive value for bevacizumab treatment.
Noninvasive characterization of glioblastoma using MR imaging has been extensively studied to predict the treatment effect and subsequent patient survival in patients with recurrent glioblastoma treated with bevacizumab. Focused sequences included perfusion MR imaging,8,21⇓⇓-24 31P MR spectroscopy,25,26 and ADC.9⇓⇓⇓⇓⇓⇓⇓⇓-18 Choi et al8 demonstrated that the pooled HRs between responders and nonresponders to bevacizumab, as defined by the relative CBV on dynamic susceptibility contrast MR imaging, were 0.47 (95% CI, 0.29–0.76) for OS and 0.46 (95% CI, 0.28–0.76) for PFS, indicating that tumor perfusion was decreased in responders, resulting in longer survival. The results of their meta-analysis showed the utility of perfusion MR imaging in patients with recurrent glioblastoma being treated with bevacizumab, though the timing of perfusion MR imaging differed among the studies (ie, changes of relative CBV values and posttreatment relative CBV were assessed simultaneously). The results in the present study may be more uniformly applicable to individual cases, given that all the timing of ADCL values was during pretreatment.
The biologic mechanisms of the worse survival in patients with tumors showing a low ADCL have not been pathologically proved. One hypothesis is that a more hypoxic or hypercellular nature of the tumor is represented by a low ADCL, making bevacizumab treatment less effective and the tumor more aggressive, as Ellingson et al11 pointed out. Indeed, the correlation between high tumor cellularity and low ADC values is known. However, ADC values fluctuate depending on the degree of intratumoral vascular edema, cystic change, and necrosis.27 The evaluated studies in this meta-analysis excluded the areas of macroscopic cystic, hemorrhagic, and necrotic changes from the VOIs for ADC histogram analysis, but intratumoral microscopic changes and vascular edema might have affected the results.
In their study with vestibular schwannomas, Plotkin et al28 reported that the higher the pretreatment ADC value, the greater was the bevacizumab-induced tumor shrinkage, suggesting that the higher ADC was associated with a higher degree of intratumoral vascular edema, which is more likely to respond to anti-VEGF therapy. Similarly, in recurrent glioblastoma, intratumoral vascular edema and microscopic cystic/necrotic changes, as well as tumor cellularity, might have affected the ADC values. To elucidate this, further studies with radiopathologic correlation will be necessary.
Not only for patients with recurrent glioblastoma but also for those with newly diagnosed glioblastoma, the prognostic performance of pretreatment ADCL has been reported.29⇓-31 Notably, these studies showed opposite results from each other as well as from this study; ie, the study by Wirsching et al29 demonstrated that a longer OS was associated with a higher ADCL, whereas the other 2 studies30,31 reported a trend in which longer OS was associated with a lower ADCL or a lower mean ADC, respectively. It remains to be seen whether the differences in the results among these studies and the present study reflect differences in the nature of newly diagnosed and recurrent glioblastoma and/or the effect of heterogeneity exaggerated by the limited number of studies. Further studies of newly diagnosed glioblastoma treated with bevacizumab and its relationship with pretreatment ADC value are warranted to clarify this issue.
There are some limitations to this study. First, like all meta-analyses, the conclusions of this study are limited by the heterogeneity of the included studies, such as patient age, the regimen, and different factors used in the multivariate analyses, though the results of Cochran Q tests and I2 values for OS and PFS indicated low heterogeneity, implying that the pooled results were robust. Second, the definition of the recurrence of glioblastoma varied among the included studies. Third, the definition of glioblastoma could be different from that of the 2021 World Health Organization Classification.32 Further investigation is needed to determine the role of ADCL in glioblastoma in the new definition. Third, unclear risk of bias remained in 1–2 studies in terms of patient selection, index test, and reference standard. Finally, the methodology of the meta-analysis was limited by the inability to obtain 1 potentially relevant reference for a full-text review.
CONCLUSIONS
The systematic review and meta-analysis of this study support the prognostic value of ADCL in patients with recurrent glioblastoma treated with bevacizumab, with a low ADCL demonstrating decreased OS and PFS. On the other hand, the role of ADCL as a predictive imaging biomarker was not confirmed.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received September 8, 2021.
- Accepted after revision November 15, 2021.
- © 2022 by American Journal of Neuroradiology