Abstract
BACKGROUND AND PURPOSE: There have been growing concerns around potential risks related to sports-related concussion and contact sport exposure to repetitive head impacts in young athletes. Here we investigate WM microstructural differences between collegiate football players with and without sports-related concussion.
MATERIALS AND METHODS: The study included 78 collegiate athletes (24 football players with sports-related concussion, 26 football players with repetitive head impacts, and 28 non-contact-sport control athletes), available through the Federal Interagency Traumatic Brain Injury Research registry. Diffusion metrics of diffusion tensor/kurtosis imaging and WM tract integrity were calculated. Tract-Based Spatial Statistics and post hoc ROI analyses were performed to test group differences.
RESULTS: Significantly increased axial kurtosis in those with sports-related concussion compared with controls was observed diffusely across the whole-brain WM, and some focal areas demonstrated significantly higher mean kurtosis and extra-axonal axial diffusivity in sports-related concussion. The extent of significantly different WM regions decreased across time points and remained present primarily in the corpus callosum. Similar differences in axial kurtosis were found between the repetitive head impact and control groups. Other significant differences were seen at unrestricted return-to-play with lower radial kurtosis and intra-axonal diffusivity in those with sports-related concussion compared with the controls, mainly restricted to the posterior callosum.
CONCLUSIONS: This study highlights the fact that there are differences in diffusion microstructure measures that are present not only between football players with sports-related injuries and controls, but that there are also measurable differences between football players with repetitive head impacts and controls. This work reinforces previous work showing that the corpus callosum is specifically implicated in sports-related concussion and also suggests this to be true for repetitive head impacts.
ABBREVIATIONS:
- AK
- axial kurtosis
- AWF
- axonal water fraction
- CC
- corpus callosum
- Daxon
- intra-axonal diffusivity
- De,‖
- extra-axonal axial diffusivity
- De,⊥
- extra-axonal radial diffusivity
- DKI
- diffusional kurtosis imaging
- FA
- fractional anisotropy
- HC
- healthy controls
- MD
- mean diffusivity
- MK
- mean kurtosis
- RHI
- repetitive head impact
- RK
- radial kurtosis
- SCAT3
- Sport Concussion Assessment Tool 3
- SRC
- sports-related concussion
- TBSS
- Tract-Based Spatial Statistics
- WMTI
- white matter tract integrity
Traumatic brain injury is a known risk factor for a host of neurodegenerative disorders,1 and in recent times, there have been growing concerns around potential risks related to sports-related concussion (SRC) and contact sport exposure to repetitive head impacts (RHIs) in our young athletes. There is mounting evidence that concussion and RHI in contact-sport athletes may predispose to long-term consequences, including serious neurologic sequelae such as chronic traumatic encephalopathy despite the lack of macroscopic brain injuries.2,3 It is, thus, of increasing importance to understand the effects that SRC and RHI may have on brain tissue microstructure.
Advanced MR imaging provides one of the few windows into the human brain for detection of injury relating to concussion. In particular, investigators have used DTI to assess WM integrity in athletes with SRC, showing areas of abnormal WM fractional anisotropy (FA) and mean diffusivity (MD).4,5 While DTI assumes Gaussian diffusion in biological tissue, diffusional kurtosis imaging (DKI)6 has been used to detect non-Gaussian diffusion behavior as a reflective marker for microstructural heterogeneity and complexity of tissue, providing improved sensitivity to tissue microstructure. Increased kurtosis has been shown after SRC compared with matched contact-sport control athletes without SRC.7 Given the inherently nonspecific nature of the empiric measures of DTI and DKI, more recent work has been done to study non-sports-related mild traumatic brain injury using compartment-specific WM models8 in an attempt to describe biophysically meaningful microstructure after injury.9,10 Specifically, WM tract integrity (WMTI) metrics, including axonal water fraction (AWF), intra-axonal diffusivity (Daxon) along axons, and extra-axonal axial (De,‖) and extra-axonal radial (De,⊥) diffusivities, can be derived from a straightforward, recently described standard model of multishell diffusion imaging to disentangle intra- and extra-axonal environments.8 Several studies including animal validation11 and in vivo human studies12⇓⇓-15 have shown that WMTI metrics add specificity regarding underlying tissue microstructure to the empiric diffusion measures such as FA.
In this study, we hypothesized that there are subtle changes due to SRC as well as RHI exposure detectable with advanced diffusion imaging. Specifically, we sought to determine what, if any, effects there may be from SRC as well as RHI exposure encountered through routine collegiate-level contact-sport participation on brain microstructure. The data are available through the Federal Interagency Traumatic Brain Injury Research registry from the National Collegiate Athletic Association–Department of Defense Concussion Assessment, Research and Education (CARE) Consortium.16
MATERIALS AND METHODS
Study Population
Available data through the Federal Interagency Traumatic Brain Injury Research registry from the National Collegiate Athletic Association-Department of Defense Concussion Assessment, Research and Education (CARE) Consortium study were used in this study.16 Participant school-level institutional review board approval and participant consent were obtained. Athletes with SRC within 48 hours and those without SRC during the 2016 to 2018 football seasons were studied. Inclusion criteria for this study were male football players as well as asymptomatic, non-contact-sport control athletes who had available multishell diffusion MR imaging data obtained on a 3T Magnetom Prisma scanner (Siemens). Scanner type was restricted to minimize data variability attributable to scanner hardware and software.
We studied a total of 78 collegiate athletes: 1) 24 football players within 48 hours of SRC (19.7 [SD, 1.1] years of age), 2) twenty-six football players with RHI exposure without a history of SRC during the study period (19.3 [SD, 1.2] years of age), and 3) twenty-eight non-contact-sport control athletes without RHI or a history of SRC (healthy controls [HC]; 19.7 [SD, 1.4] years of age). For all groups, symptom severity was assessed using the Sport Concussion Assessment Tool 3 (SCAT3). The non-contact-sport control group comprised asymptomatic (SCAT317 < 10)18 non-contact-sport control male athletes. Non-contact-sport controls were members of the baseball (n = 17), field event (n = 2), and cross-country (n = 9) teams. Longitudinal recovery trajectories for the SRC group were studied using the following 4 time points: 1) 24–48 hours after injury, 2) asymptomatic and after passing initial clearance to begin the return-to-play protocol (asymptomatic), 3) seven days following unrestricted return-to-play, and 4) six months after injury. For comparison, the RHI and HC groups underwent MR imaging at similar time intervals matched with the subjects with SRC.
MR Imaging Acquisition
Details of the MR imaging parameters can be found in Broglio et al.16 In brief, MR images included here were all acquired on 3T Magnetom Prisma scanners. Diffusion imaging was performed with the following parameters: b-values = 1000, 2000 s/mm2, 30 diffusion directions, 8 b=0 images, FOV = 243 × 243 mm, matrix = 90 × 90, slices = 64, isotropic resolution = 2.7 mm, TR/TE = 7900/98 ms.
Image Analyses
Diffusion Image Processing.
Preprocessing steps included Marchenki-Pastur principal component analysis (MP-PCA) denoising,19 Gibbs correction,20 eddy current distortion, and motion correction21 and outlier detection.22 In-house image-processing software developed in Matlab R2019b (MathWorks) was used to generate parameter maps of DTI (FA, mean/axial/radial diffusivities), DKI (mean/axial/radial kurtosis [MK/AK/RK]), and WMTI metrics (AWF, Daxon, De,‖, De,⊥).
Tract-Based Spatial Statistics
Tract-Based Spatial Statistics (TBSS) was used for voxelwise analyses.23 Briefly, individual FA maps were affine-transformed to the FA template (Montreal Neurological Institute 152 space), and voxelwise statistics were performed on maximum FA values projected onto the WM skeleton. All parametric maps underwent unified transformations and processes. The WM skeleton was thresholded at FA = 0.2 for DTI and DKI metrics. For WMTI metrics, we restricted analysis to WM regions consisting primarily of single-fiber orientations (FA threshold = 0.4), as recommended.8
ROI Analyses.
Post hoc subanalyses were performed in an attempt to separate the effects of SRC from RHI. Specifically, the corpus callosum (CC) was studied to understand the temporal evolution of SRC changes. ROIs of the genu, body, and splenium were generated on the basis of the ICBM-DTI-81 WM atlas labels24 by nonlinearly registering each subject's FA map to the FA template.21 A reversed warping procedure was performed to assign the atlas labels to each subject's space.21 All ROIs were manually reviewed and edited if needed. For each ROI, the mean value was obtained only in voxels with FA ≥ 0.2 for DTI and DKI metrics and with FA ≥ 0.4 for WMTI metrics, as recommended.8
Statistical Analyses
ANOVA was used to test group differences of SCAT3 total symptom severity scores as well as mean values within the ROIs at each time point with a Tukey post hoc pair-wise test for multiple comparisons using SPSS, Version 25 (IBM). Results were considered significant for P < .05, corrected for multiple comparisons. For TBSS, statistical tests were conducted with 5000 random permutations to identify statistically significant effects among groups.25 The statistical significance level was set at P < .05, corrected for multiple comparisons by controlling the family-wise error rate with the threshold-free cluster enhancement option.26
RESULTS
The Online Supplemental Data summarize the characteristics of the study cohorts across time points. Briefly, the average time since injury was 8.1 (SD, 5.6) days to an asymptomatic state (time point 2) and 27 (SD, 12.5) days to unrestricted return-to-play (time point 3). Subjects with SRC had significantly higher SCAT3 total symptom severity scores than both the RHI and HC groups at time point 1 (Tukey test, P < .001), but there were no significant differences at other time points. No gross intracranial abnormalities were detected. We found no significant differences between the SRC and RHI groups from both TBSS and ROI analyses.
Football Players with SRC versus Non-Contact-Sport Healthy Controls
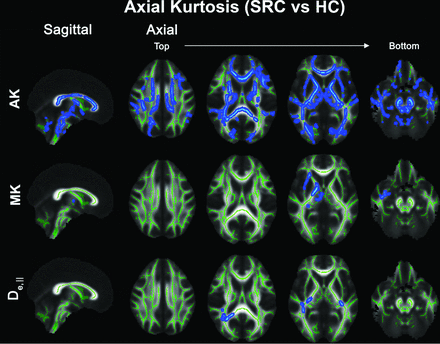
TBSS analysis revealed diffusely higher AK in athletes with SRC compared with the HC group at time point 1 (Fig 1). Additionally, there were some focal areas that demonstrated significantly higher MK and De,‖ in the SRC group: namely, the middle-posterior WM regions including the external capsule, posterior limb of the internal capsule, cerebral peduncle, uncinate fasciculus, inferior longitudinal fasciculus, posterior thalamic radiation, retrolenticular part of internal capsule, and splenium of the CC. The extent of WM regions that showed significant differences in AK between the SRC and HC groups decreased by the fourth time point (6-month follow-up) (Fig 2), though remains present, located almost entirely within the CC (Fig 2A). In addition, significantly different WM regions in MK and De,‖ observed at time point 1 (Fig 1) were not seen at later time points. The other significant between-group differences were seen at unrestricted return-to-play (time point 3) with lower RK and Daxon in the SRC group compared with the HC group, mainly restricted to the posterior callosum (Online Supplemental Data). The results of the ROI analyses in the CC regions were consistent with the TBSS results, showing significantly higher AK across time points (Tukey test, P < .05). Moreover, significantly lower RK (Tukey test, P = .022) in the body of the CC and significantly lower Daxon (Tukey test, P = .045) in the body and splenium of the CC in the SRC group were present only at time point 3 compared with the HC group, supporting the TBSS results (Online Supplemental Data).
TBSS results comparing SRC and HC groups at time point 1 (24–48 hours postinjury for SRC; baseline for HC). Top, significant voxels (blue) demonstrating increased AK in the SRC group are present diffusely throughout the WM. Increased MK (middle) and De,‖ (bottom) in the SRC group are also seen in more focal areas of middle posterior WM regions including the external capsule, posterior limb of the internal capsule, cerebral peduncle, uncinate fasciculus, inferior longitudinal fasciculus, posterior thalamic radiation, retrolenticular part of internal capsule, and splenium of the corpus callosum. The significance level was P < .05, corrected for multiple comparisons by controlling the family-wise error rate. Significant voxels were enlarged using TBSS fill (FSL command; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS/UserGuide#Displaying_TBSS_Results) for illustrative purposes and were overlapped onto the mean FA skeleton (green).
Evolution of differences in AK between the SRC and HC groups across 4 time points. A, Maps show regions along the WM skeleton that demonstrate a significant difference (blue) in AK between the SRC and HC groups across 4 time points (T1–T4). B, Corresponding bar graphs show the percentage of significantly different voxels on the skeleton across time points. The extent of significantly different WM regions decreases across time points, remaining present primarily within the corpus callosum at time point 4.
Nonconcussed Football Players versus Non-Contact-Sport Healthy Controls
We found widespread differences in AK between the RHI and HC groups as well as diffuse differences in MK, with higher kurtosis measures in RHI (Fig 3). In addition, focal areas demonstrated higher De,‖ and AWF in the RHI group compared with the HC group, including the CC, superior/anterior/posterior corona radiata, superior longitudinal fasciculus, posterior limb of the internal capsule, retrolenticular part of the internal capsule, posterior thalamic radiation, and cerebral peduncle. The ROI analysis results were also in accordance with the TBSS results, showing significantly higher MK (Tukey test, P = .047) as well as a trend toward higher AK in the genu (Tukey test, P = .098) and splenium (Tukey test, P = .053). Significantly higher De,‖ in the body of the CC (Tukey test, P = .016) and a trend toward higher AWF in the splenium (Tukey test, P = .067) were also observed in the RHI group compared with the HC groups.
TBSS results comparing the RHI and HC groups (time point 1): Clusters of voxels (blue) demonstrating significantly higher AK and MK in the RHI group compared with the HC group are also present diffusely across the entire WM. Additionally, increased De,‖ and AWF in the RHI group are seen in focal areas of WM regions including the corpus callosum, corona radiata, superior longitudinal fasciculus, posterior limb of the internal capsule, retrolenticular part of the internal capsule, posterior thalamic radiation, and cerebral peduncle. The significance level was P < .05, corrected for multiple comparisons by controlling the family-wise error rate.
DISCUSSION
In this study, we demonstrate extensive and diffuse WM differences in diffusion metrics, particularly AK, not only between concussed football athletes within 48 hours of injury (SRC) and baseline non-contact-sport control subjects (HC), but also related and widespread differences between nonconcussed football athletes (RHI) and HC subjects. The findings suggest that some of the diffuse measurable microstructural changes observed in the SRC group may relate not to sports-related concussion but to a background of exposure to repeat subconcussive head impacts. Similar changes of kurtosis have been reported previously in nonconcussed young football players with cumulative head impact exposure.27 In addition, the affected WM regions in the SRC group compared with non-contact-sport controls decreased across time points, suggesting partial recovery of microstructural changes during the study period (Fig 2B). Most interesting, persistent higher AK in the SRC group was observed compared with the non-contact-sport controls mainly in the CC on 6-month follow-up scans (Fig 2A), suggesting longer-term persistence of microstructural changes associated with SRC. Although AK is not specific, it reflects tissue microstructural complexity along the long axis of the axon and has been previously associated with changes such as reactive astrogliosis28 as well as frank axon damage.13
We observed few scattered, focal areas of higher De,‖ in the SRC group compared with the non-contact-sport controls only at the acute stage (Fig 1); however, in the RHI group, higher De,‖ was persistently observed across all time points compared with the non-contact-sport controls (Online Supplemental Data). Previous studies have reported De,‖ to be sensitive to changes along the axons in the extra-axial space due to gliosis, loss of oligodendrocytes, extracellular inflammation, and vasogenic edema.15 Of note, these individuals with RHI, unlike the players with SRC who were taken out of active play, were exposed to repeat head impacts throughout the study period. Our findings of the elevated De,‖ accompanied by the increases in AK and MK in these individuals indicate the presence of complex microstructural alterations, localizing to the extracellular space, that seem to relate to exposure to repeat head impacts. While similar findings were present at the initial time point in the SRC group, these were no longer present at later time points after a recuperation period without RHI exposure, suggesting that at least some of the white matter microstructural changes associated with RHI exposure may be reversible early on.
We also observed decreased RK and Daxon and increased AK in the SRC group compared with non-contact-sport controls, present only at time point 3 when the players with SRC were cleared to return to play. These observations were present mainly in the midposterior CC (Online Supplemental Data). Both increased AK and decreased RK have previously been reported by Zhuo et al28 in a controlled compact injury rat model, similarly in the subacute stage after injury (7 days postinjury). These findings support the notion that some changes may become apparent in the subacute phase after the initial insult and that diffusion characteristics relating to the intra-axonal compartment itself are affected. Changes in Daxon have been attributed to a range of pathologies, including axonal disruption as well as axonal injuries such as beading, varicosity, undulation, and swelling.29⇓-31 Decreases in Daxon have previously been observed in the setting of ischemic brain injury13 as well as in subacute civilian, non-sports-related mild traumatic brain injury.9
From biomedical modeling of torsional and stretch forces after head impact, it is known that the CC is an at-risk structure.32,33 Our findings are in keeping with prior evidence that the CC is one of the most affected WM regions after SRC, and this result was also the case in our group exposed to RHI.5,9,34,35 The CC is the largest transhemispheric WM structure, composed of several important anatomically and functionally distinct WM tracts. Our results reinforce the idea that WM injury, particularly affecting the CC, is mechanistically important in SRC and also suggest that this is important in RHI as well.
Our findings add to previously published results from a larger cohort from the National Collegiate Athletic Association-Department of Defense Concussion Assessment, Research and Education (CARE) Consortium study.4,5,36 The current study focuses on diffusion MR imaging microstructural changes; specifically here, we study the subset of the total cohort who underwent multishell diffusion MR imaging accommodating compartment diffusion modeling as discussed in the Materials and Methods section. We also restricted our study by scanner type and sport to minimize data variability relating to these factors.37,38 Moreover, the main difference between the current study and previous studies4,5,36 is that we have used compartment-specific diffusion parameters to try to understand potential changes in the underlying tissue microstructure. Of note, in this restricted cohort, we found no significant differences in conventional DTI metrics such as FA or MD that were present in previous studies, possibly due to use of a smaller subset of the cohort.39
There are several limitations to this study. First, study time points were based on symptomatology and clinical status rather than a predefined follow-up interval, prohibiting parsing of the precise temporal evolution of diffusion MR imaging changes relating to injury and/or exposure. The range in the length of the symptomatic period after injury is, however, reflective of the natural history of SRC. Second, the study includes relatively small sample sizes, which can reduce statistical power, particularly at later time points (time points 3 and 4). The smaller cohort size may have also explained why we did not see changes in FA and MD, which others have previously shown.4,5,36 Finally, we used standard TBSS methods that are well-documented, but TBSS is sensitive to maximal deviations in diffusion metrics because of the use of maximum values projected onto the white matter skeleton. The benefit of such an approach is that it reduces the need for image smoothing and alleviates any residual misalignment.40 In this study, we used both TBSS and post hoc ROI analyses to assess WM changes to look for consistencies between the methods.
CONCLUSIONS
There are differences not only in concussed football athletes but also in nonconcussed football athletes compared with non-contact-sport control athletes in terms of diffusion microstructure measures. These findings reinforce previous work showing that the corpus callosum is specifically implicated in football athletes with SRC and also suggest this to be true for football athletes with RHI. Further study on the effect of RHI across time may provide insight into the temporal dynamics of injury in both SRC and athletes who may be exposed to RHI.
Footnotes
This work was supported by the National Institutes of Health grant Nos. NIH R01 NS119767-01A1, R01 NS039135-11, R21 NS090349, R56 NS119767, P41 EB017183; the Department of Defense, grant No. DoD PT190013; and the Leon Lowenstein Foundation.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received January 6, 2022.
- Accepted after revision April 4, 2022.
- © 2022 by American Journal of Neuroradiology