Abstract
BACKGROUND AND PURPOSE: IDH and TERT mutations might infiltratively manifest within normal-appearing white matter with specific phenotypes such as microstructural changes undetectable by standard MR imaging contrasts but potentially associable with DTI variables. The aim of this retrospective glioma study was to statistically investigate IDH and TERT associations and classifications with DTI reported microstructure in normal-appearing white matter.
MATERIALS AND METHODS: Retrospective data from patients imaged between March 2012 and February 2016 were analyzed by grouping them as IDH–TERT subgroups and by IDH and TERT mutation status. DTI variables in the IDH-TERT subgroups were first identified by the Kruskal-Wallis test, followed by Dunn-Šidák multiple comparisons with Bonferroni correction. IDH and TERT mutations were compared with the Mann-Whitney U test. Classification by thresholding was tested using receiver operating characteristic analysis.
RESULTS: Of 170 patients, 70 patients (mean age, 43.73 [SD, 15.32] years; 40 men) were included. Whole-brain normal-appearing white matter fractional anisotropy (FA) and relative anisotropy (RA) (P = .002) were significantly higher and the contralateral-ipsilateral hemispheric differences, ΔFA and ΔRA, (P < .001) were significantly lower in IDHonly patients compared with TERTonly, with a higher whole-brain normal-appearing white matter FA and RA (P = .01) and ΔFA and ΔRA (P = .002) compared to double positive patients. Whole-brain normal-appearing white matter ADC (P = .02), RD (P = .001), λ2 (P = .001), and λ3 (P = .001) were higher in IDH wild-type. Whole-brain normal-appearing white matter λ1 (AD) (P = .003), FA (P < .001), and RA (P = .003) were higher, but Δλ1 (P = .002), ΔFA, and ΔRA (P < .001) were lower in IDH mutant versus IDH wild-type. ΔFA (P = .01) and ΔRA (P = .02) were significantly higher in TERT mutant versus TERT wild-type.
CONCLUSIONS: Axial and nonaxial diffusivities, anisotropy indices in the normal-appearing white matter and their interhemispheric differences demonstrated microstructural differences between IDH and TERT mutations, with the potential for classification methods.
ABBREVIATIONS:
- AD
- axial diffusivity
- AUC
- area under the curve
- DAI
- diffusion anisotropy indices
- DN
- double negative
- DP
- double positive
- FA
- fractional anisotropy
- HMeD
- hemispherical mean differences
- LBTh
- lower bound thresholding
- mut
- mutant
- NAWM
- normal-appearing white matter
- NPV
- negative predictive value
- PPV
- positive predictive value
- RA
- relative anisotropy
- RD
- radial diffusivity
- ROC
- receiver operating characteristic
- UBTh
- upper bound thresholding
- WB
- whole brain
- wt
- wild-type
Glioma is the most common CNS tumor with overall survival ranging from 12 to 15 months to several years, depending on tumor severity.1 Recently, genotype information in clinical workflow proved to be valuable2 and was integrated into the WHO classification.3 Therein, isocitrate dehydrogenase (IDH) mutation is associated with longer overall survival, around 57 months, and is commonly seen in low-grade (grade 2, grade 3) gliomas.2,4-6 Telomerase reverse transcriptase (TERT) mutation presents with a more aggressive disease course, eg, with neutrophil infiltration,7 leading to a lower overall survival of 11.5 months, mostly in high-grade (grade 4) tumors.2,8 Remarkably, overall survival increases to 125 months when the tumor is both IDH- and TERT-mutated.6
When identifying genotypes, biopsy might miss relevant loci due to the notorious heterogeneity of gliomas.9 Complete organ coverage by MR imaging might provide a more accurate assessment, especially by analyzing the normal-appearing white matter (NAWM) for the diffuse properties of the disease such as infiltrative tendencies to migrate through white matter fibers.10,11
The migration process involves complex interactions with extracellular matrix components and cytokines, which, in turn, lead to displacement or, for grade 4, destruction of the white matter fibers.12 T1WI and T2WI might not be sufficiently sensitive to NAWM infiltration,13 whereas microstructural changes such as neoplastic angiogenesis and elevated cellular density14,15 are investigated with DTI and quantified by its eigenvalues and diffusion anisotropy indices (DAIs).16
In the past, decreased relative anisotropy (RA) found in the NAWM of patients with high-grade gliomas,17 and increased ADC coupled with decreased N-acetylaspartate in the contralateral NAWM suggested microstructural damage possibly caused by infiltrating tumor cells.18 Tumor grades histopathologically report microstructure;19 so low-grade and, therefore, IDH mutated (IDHmut) tumors exhibit higher diffusion anisotropy and lower ADC in the NAWM compared with high-grade17,20 and IDH wild-type (wt)21 tumors. In addition, DAI changes in the distal NAWM have been reported for different glioma grades.22
Complementing tumor-site-focused past studies by investigating regions outside the tumor might potentially be relevant because it would inform on the diffuse nature of the disease. The aim of this study was to investigate microstructural changes in the NAWM by analyzing comprehensively the DTI variables within the whole brain (WB) and between “healthy” and “pathologic” hemispheres, for statistically associating them with IDH and TERT mutations.
MATERIALS AND METHODS
Clinical Data
In this institutional review board–approved retrospective study, the cohort was gathered without recruitment from existing data of clinical patients who had consented. Of 170 consecutive patients with gliomas treated at Acıbadem Hospitals (Istanbul, Turkey) between March 2012 and February 2016, with written informed consent, 54 without T2WI and/or DTI, 30 postoperative, 2 with unavailable mutation data and 11 with unavailable TERT status, 2 with conflicting genetic results, and 1 with a suboptimal NAWM DTI mask were excluded (Fig 1), leaving 70 patients (mean age, 43.73 [SD, 15.32] years of age; female/male ratio: 30/40), with 1 grade 1, 29 grade 2, 22 grade 3, and 18 grade 4 gliomas and 41 patients with IDHmut (29 IDHwt); 37 patients were TERTmut (33 TERTwt).
Patient-selection flowchart.
Histopathologic analysis was performed on surgically removed tumor samples. IDH and TERT mutations were determined using either minisequencing or Sanger sequencing. Patients were stratified into 4 molecular subgroups, hereafter referred to as IDH-TERT subgroups (Table 1):
Double negative (DN): IDHwt, TERTwt (n = 9).
IDHonly: IDHmut, TERTwt (n = 24).
TERTonly: IDHwt, TERTmut (n = 20).
Double positive (DP): IDHmut, TERTmut (n = 17).
Cohort characteristics
MR Imaging Data
MR imaging was performed on a 3T Magnetom Tim Trio MR imaging scanner (Siemens) with a 32–channel head coil, 1–7 days before the operation. The scanning protocol included T2WI acquired using a 2D turbo spin-echo sequence with voxel dimensions of 0.26 × 0.26 × 0.26 mm with 20 axial slices, TE/TR = 107/3470 ms, slice thickness/spacing = 5/6.5 mm, flip angle = 120°. DTI data were acquired using a 2D diffusion EPI sequence with 1.8 × 1.8 × 1.8 mm voxels, 60 axial slices, TE/TR = 107/3470 ms, slice thickness/spacing = 1.8/2.34 mm, flip angle = 90°, and b-value = 1000 ms/mm2 with 20 diffusion gradient vectors. Eigenvalue maps for the 3 eigenvalues (λ1≥λ2≥λ3) were computed by the console computer of the scanner.
All image volumes were transferred in DICOM format, then anonymized using in-house-developed scripts based on the Grassroots DICOM library23 and were converted to Neuroimaging Informatics Technology Initiative (NIfTI) format using Medical Image Processing, Analysis, and Visualization software (MIPAV; http://mipav.cit.nih.gov).24
NAWM Segmentation and Coregistration
NAWM regions were selected on T2WI using semiautomatic level set segmentation tools in MIPAV by 2 trainees with 2 years of experience OG and OA, inspected by an imaging scientist with 25+ years of experience AÖ, and approved by a neuroradiologist with 30+ years of experience, AD.
Images obtained without diffusion weighting (B0 images) were coregistered onto T2WI with the FSL software package (http://www.fmrib.ox.ac.uk/fsl)25 using the mutual information cost function with the trilinear interpolation method. The transformation optimizing the B0→T2WI coregistration problem was applied for coregistering eigenvalue maps onto T2WI by interpolating with the nearest neighbor method (Fig 2).
NAWM segmentations and masks. T2WI (A), semiautomatic NAWM boundary demarcations overlaid on T2WI (B), the coregistered B0 image (C), NAWM boundary demarcations on the coregistered B0 image (D), total NAWM mask on T2WI (E), on B0 image (F), and the corresponding right (G) and left (H) hemisphere masks.
The distortion of NAWM masks caused by the difference of total slice numbers among the MR imaging modalities while coregistering was corrected with an in-house-developed Matlab (MathWorks) code (KP, 2+ years of experience), which identifies shared pixel coordinates in matching B0 images mapped to T2WI and the original B0 image. The code infers mask pixels in the “sandwich” slices from the “shell” slices.
Furthermore, an in-house-developed (KP, 2+ years of experience) Matlab code subdivided the mask images automatically into left and right hemispheres, which were used for computing contralateral and ipsilateral DAI and eigenvalue distributions (Fig 2). Quality assurance for the image-processing routines was conducted by HH, SK and KP, each with 2+ years of experience, and inspected and approved as aforementioned.
Variables
For each patient, DAIs (Online Supplemental Data) were computed from the eigenvalues (λ1≥λ2≥λ3) on WB, contralateral, and ipsilateral NAWM pixels. For each patient, each variable’s mean over WB, MeanWB, and the hemispheres, Meancontralat, Meanipsilat, were calculated. Each patient’s ipsilateral hemisphere mean was subtracted from the contralateral hemisphere mean for obtaining in-patient hemispherical mean differences (HMeD) of DAIs and eigenvalues:
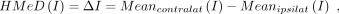 where Ι denotes any of the DTI variables. All of the computations were implemented with in-house-developed Matlab code (HH, 2+ years of experience).
where Ι denotes any of the DTI variables. All of the computations were implemented with in-house-developed Matlab code (HH, 2+ years of experience).
Statistical Analysis
Statistical power calculations indicate that with the exception of ADC, whose results were nevertheless reported herein, all of the variables had acceptable statistical power for the number of included patients (Online Supplemental Data).
The Kruskal-Wallis test with a P < .05 threshold was used for testing whether DAIs and eigenvalues could detect differences among IDH-TERT subgroups. Variables with statistical significance were then subjected to a Dunn–Šidák multiple comparison analysis with pair-wise subgroup comparisons for identifying differentiating variables. Subsequently, P values of multiple comparison analysis were adjusted with the Bonferroni correction (P < .008). Anisotropy differences between IDH and TERT mutation status were compared using the Mann-Whitney U test with a threshold of P < .05.
Statistical analysis was performed with Matlab Statistics and Machine Learning Toolbox (https://www.mathworks.com/products/statistics.html) by HH under the supervision of AÖ with 6+ and 25+ years of experience, respectively.
Classification by Thresholding
For investigating genotype classification as proof of principle, decision by thresholding was tested on each variable for separating each mutation group from the rest. Classifier performance was investigated with the receiver operating characteristic26 (ROC) curve’s area under the curve (AUC)26 (with AUC = 1 as the best performance indicator) and positive and negative predictive values (PPV, NPV) at the optimal operating threshold identified by determining on each ROC curve the closest point to the top left corner (False Positive Rate = 0, True Positive Rate = 1), corresponding to sensitivity = 1 and specificity = 1 (Online Supplemental Data).
The steps were applied using each threshold as a lower bound (Value ≥ Threshold) and as an upper bound (Value ≤ Threshold). Therein, AUC values for these decision criteria add up to 1, leading to select the thresholding criterion (upper or lower bound) with a higher AUC for a given variable. By contrast, an AUC close to 0.5 indicates poor classification capability of the variable (Online Supplemental Data).
ROC analysis and reporting were implemented with in-house Matlab code developed by AÖ with 25+ years of experience.
RESULTS
IDH–TERT Subgroups
Whole Brain.
Among IDH-TERT subgroups, in the WB-NAWM, there was a statistically significant difference in all DAIs and eigenvalues (P < .05), with the exception of ADC (P = .10 for WB–NAWM ADC) (Table 2). In pair-wise IDH-TERT subgroup comparisons, WB-NAWM radial diffusivity (RD) was higher in TERTonly patients compared to IDHonly (P = .01), with higher values in TERTonly compared to DP patients (P = .04) (Fig 3). WB-NAWM fractional anisotropy (FA) and RA were significantly higher in IDHonly patients compared to TERTonly (P = .002 for both), with a higher WB-NAWM FA and RA compared to DP (P = .01 for both). WB-NAWM axial diffusivity (AD) had higher values in the IDHonly subgroup compared with TERTonly (P = .04), whereas WB-NAWM λ2 had significantly higher values (P = .007) and WB-NAWM λ3 showed higher values (P = .01) in the TERTonly gliomas compared to IDHonly. WB-NAWM λ3 also had higher values in TERTonly patients compared to DP (P = .03).
Boxplots of WB-NAWM means of ADC, λ1, RD, λ2, λ3, FA, and RA grouped by IDH-TERT subgroups (A), IDH mutation (B), and TERT (C) mutation statuses, respectively. The asterisk indicates P < .05, and double asterisks indicate P < .008 according to the Bonferroni correction for multiple-comparison analysis. The plus sign indicates outlier values, whiskers indicate minimum and maximum values, the box limits are 25th and 75th percentiles, and the midline shows the median. Neg. indicates negative; pos., positive.
Comparison of WB-NAWM means and HMeD of diffusion parameters for IDH-TERT molecular subgroups
Interhemispheric Differences.
When comparing hemispheres within IDH-TERT subgroups, there was a statistically significant difference in the interhemispheric mean differences of all DAIs and eigenvalues (P < .05), with the exception of ADC (P = .10 for ΔADC) (Table 2). With the exception of DN and DP groups, ΔADC distributions completely shifted to negative values, indicating that in the presence of only a single mutation, ADC values on the “healthy” (contralateral) side tend to be lower than those of the tumor (ipsilateral) side.
For all IDH-TERT subgroups, λ2, λ3, and thus RD had larger values ipsilaterally. TERTonly had the largest shift, followed by IDHonly. HMeD medians in DN and DP were negative but closer to zero. With the exception of λ1 (AD) of the DN, all the diffusivities had higher values ipsilaterally for the IDH-TERT subgroups. This finding might potentially indicate an overall disruption of the microstructure in the tumor hemisphere.
For TERTonly and DP, the median of Δλ1 was negative but closer to zero, indicating a higher λ1 ipsilaterally. Δλ1 was lower in IDHonly gliomas compared to DN (P = .02). DN and TERTonly distributions point to higher contralateral anisotropy significantly in TERTonly and mildly in DN. ΔRD and Δλ2 had higher values in DN patients in comparison to TERTonly (P = .04 for both).
Distributions of ΔFA and ΔRA from IDHonly and DP had median values close to zero, indicating anisotropy resemblance of the hemispheres for these mutations. ΔFA and ΔRA were significantly higher in TERTonly gliomas compared to IDHonly (P < .001 for both) and DP (P = .002 for both) (Fig 4 and Table 2). Lastly, distributions of DN and TERTonly point to higher contralateral anisotropy significantly in TERTonly and mildly in DN.
IDH and TERT Mutation Status
Whole Brain.
Among IDHwt and IDHmut gliomas, WB-NAWM ADC (P = .02), RD (P = .001), λ2 (P = .001), and λ3 (P = .001) were higher in the IDHwt group (Table 3). In contrast, WB-NAWM λ1 (P = .003), FA (P < .001), and RA (P = .003) were higher in IDHmut gliomas compared to IDHwt.
Comparison of WB–NAWM means and HMeD of diffusion parameters for IDH and TERT mutation status
When TERTwt and TERTmut gliomas were compared, none of the WB-NAWM DAIs and eigenvalues demonstrated a statistically significant difference (P > .05).
In consequence, similar relative median levels presented in all of the variables when comparing IDHwt and TERTmut versus their respective counterparts (eg, lower AD and higher RD for IDHwt and TERTmut versus IDHmut and TERTwt, respectively) demonstrated a common phenotype for these aggressive genotypes.
Interhemispheric Differences.
For wild-type and mutant pairs, all of the diffusivity variables for all of the genotype pairs showed higher values ipsilaterally. ΔADC, ΔRD, and Δλ2 were leveled; Δλ1 differed more for IDHmut and TERTwt, and, likewise, Δλ3 for IDHwt and TERTmut. IDHmut and TERTwt did not show interhemispheric difference for any anisotropy index, whereas IDHwt and TERTmut had higher values contralaterally for RA and FA.
ΔFA, ΔRA (P < .001 for both), and Δλ1 (P = .002) were higher in the IDHwt group compared to IDHmut. ΔADC (P = .48), ΔRD (P = .32), Δλ2 (P = .73), and Δλ3 (P = .055) had no statistically significant difference among IDH mutation statuses.
With a remarkable resemblance, ΔFA (P = .01) and ΔRA (P = .02) were higher in TERTmut patients compared to TERTwt. ΔADC (P = .73), Δλ1 (P = .31), ΔRD (P = .14), Δλ2 (P = .27), and Δλ3 (P = .054) had no statistically significant difference among TERT mutation statuses.
Classification by Thresholding
Numeric results in this section are fully presented in the Online Supplemental Data.
IDH-TERT Subgroups.
For WB-NAWM, the best PPV was obtained for TERTonly by FA and RA (0.6842 for both) with upper bound thresholding (UBTh); overall, PPVs were low (minimum 0.1429 DN with RA lower bound thresholding [LBTh], and 0.1464 DN ADC with UBTh). In contrast, NPVs had higher values with the highest from 0.9355 for DN with RD LBTh. DN had higher NPVs for all variables for LBTh and UBTh, followed by DP, TERTonly, and IDHonly (Online Supplemental Data).
AUC values from WB-NAWM reported TERTonly classification as the best for RA (0.7800), FA (0.7760), and λ1 (AD) (0.7030) with UBTh and RD (0.7470), λ3 (0.7460), λ2 (0.7400), and ADC (0.670) with LBTh. DN had the worst performance with AUC values close to 0.5, which was also supported by the poor PPV performance for LBTh. λ1 (AD), FA, and RA had above-average performance on IDHonly and DP using LBTh (Online Supplemental Data).
The WB-NAWM findings are aligned with the results of the “Whole Brain” section of the “IDH-TERT Subgroups” section. Overall poor classification performance on DN agreed with its lack of a statistically significant difference from the other groups. FA and RA, which showed the most statistically significant differences among subgroups, especially for TERTonly classification (Fig 3A,-D, -G), had the best classifier performances.
Interhemispheric differences had poor PPVs for LBTh with the highest 0.7895 for ΔRA on TERTonly followed by 0.6296 for ΔFA on TERTonly, then followed by a substantial drop to 0.4324. For UBTh, ΔRA provided for TERTonly PPV = 1; however, the second highest value was 0.5667, which is a significant drop reflecting on overall performance. Interhemispheric NPVs had much higher values over different variables and genotypes, and higher values were concentrated on DN (maximum = 0.9750 from ΔADC and Δλ2 for LBTh; maximum = 0.9167 from ΔFA for UBTh), presenting with high values overall (minimum = 0.5750 for LBTh, 0.6304 for UBTh) (Online Supplemental Data).
Interhemispheric differences demonstrated the best AUC values on TERTonly from ΔFA (0.8330) and ΔRA (0.8280) with LBTh. DN has good performance for Δλ1 (ΔAD) (0.7377), ΔADC (0.7341), Δλ2 (0.7341), and ΔRD (0.7013) with LBTh. Performance on DP and IDHonly was poor with the exception of ΔFA (DP: 0.6349, IDHonly: 0.6902) and ΔRA (DP: 0.6448, IDHonly: 0.6866) performing better for both, and Δλ1 (ΔAD) (0.6975), for IDHonly (Online Supplemental Data), all using UBTh.
For interhemispheric differences, good performance of ΔFA and ΔRA for the TERTonly classification was in line with the results of the “Interhemispheric Differences” section under the “RESULTS” section (Fig 4G), while AUC values of ΔADC, ΔAD, ΔRD, and Δλ2 for classifying DN aligned with the separation of DN from the other subgroups in Fig 4A, -D.
IDH and TERT Mutation Status.
For WB-NAWM, PPVs were higher compared with NPVs. The highest PPV (0.7714) was obtained with LBTh of FA on IDHmut followed by λ1 (AD) (0.7500) and RA (0.7381), while λ2 (0.7647), RD (0.7632), ADC (0.7576), and λ3 (0.7568) also performed well using UBTh. PPVs for the TERTmut classification were modest with RD (0.6897) and FA (0.4828) providing maximum and minimum PPVs for LBTh, and λ1 (AD) (0.6563) and λ2 (0.4545), for UBTh. NPVs, on the other hand, had poor values with maximum values from RA (0.6429) using LBTh and RD (0.6250) using UBTh for IDHmut, and λ3 (0.5882) using LBTh and FA (0.5833) using UBTh for TERTmut, with minimum values from RD (0.3333) using LBTh and RA (0.2778) using UBTh for IDHmut, and RA (0.4359) using LBTh and λ2 (0.4054) using UBTh for TERTmut (Online Supplemental Data).
AUC values from WB-NAWM indicated good performance for IDHmut: FA and RA (0.7653 both) and λ1 (AD) (0.7115) for LBTh, and RD (0.7359), λ3 (0.7325), and λ2 (0.7283) for UBTh. In contrast, TERTmut had mediocre performance with λ2 (0.6331), RD (0.6200), and λ3 (0.6077) using LBTh and FA (0.6282) and RA (0.6274) using UBTh (Online Supplemental Data).
The better classifier performance of WB-NAWM on IDHmut was in line with its statistically significant difference from IDHwt present for all variables, shown in Fig 3B, -E, -H, whereas the lack of statistical significance for TERTmut depicted in Fig 3C, -F, -I reflected a mediocre classifier performance.
Interhemispheric difference PPVs had the highest values for IDHmut with ΔAD (0.8519), ΔFA (0.8250), and ΔRA (0.8158) using UBTh which agrees with Fig 4A–H where the IDHmut distributions for the aforementioned variables lie below the IDHwt distributions. These variables also had statistically significant differences. The highest PPV for TERTmut occurred for ΔRA (0.8182) followed by ΔFA (0.7778), both using LBTh, in accordance with Fig 4I, where the ΔFA and ΔRA distributions of TERTmut were placed slightly higher than TERTwt distributions; also, ΔFA and ΔRA were the only variables presenting a statistically significant difference (Fig 4C, -F, -I).
IDHmut NPVs for ΔFA and ΔRA were both equal to 1 for LBTh, which was in accordance with Fig 4H where IDHmut ΔFA and ΔRA distributions were below IDHwt distributions and presented statistically significant differences. However, the remaining NPVs were all below 0.5625 (Δλ3). The highest NPVs for TERTmut were all from LBTh for ΔAD (0.6333), ΔFA (0.6279), and ΔRA (0.6042), with ΔFA and ΔRA being the only variables with statistically significant differences in Fig 4C, -F, -I.
The AUC classifier performance agreed with the predictive value and statistical findings: ΔRA (0.7830), ΔFA (0.7788), and ΔAD (0.7149) had the best values for IDHmut all using UBTh; likewise, the best AUC values of TERTmut were ΔFA (0.6732), ΔRA (0.6618), and ΔAD (0.5717) all using LBTh.
DISCUSSION
In this study, DTI variables from the WB-NAWM of patients with gliomas and their interhemispheric differences were investigated for their association with IDH-TERT–based genotypes. DTI variables were analyzed as indicators of microstructural integrity in the NAWM for associating them as phenotypes of the mutations.
By probing the diffuse nature of the disease, which was scarcely studied in the past, this investigation complemented the studies focusing solely on the tumor region characteristics. In fact, there are very few glioma NAWM investigations with limited basis and scope: limited coverage of NAWM, relating interhemispheric FA differences to neurometabolites with contralateral FA increase,27 positively correlating ADC to tumor grades,22 and suggesting radiation-induced fiber damage for reducing interhemispheric FA difference.28 In contrast, for this study, NAWM data were obtained comprehensively from the WB, the hemispheres, and interhemispheric computations for radiogenomics analysis.
First, while lacking statistical significance in this study, if AD and λ2 can also differentiate between TERTonly and DP for a larger cohort in the future, DTI variables might be considered as markers separating TERTonly from groups containing IDHmut.
In aggressive gliomas, ie, IDHwt and its subset TERTonly, WB-NAWM exhibited higher ADC. However, this observation does not necessarily grant a relevance to WB-NAWM ADC due to the canceling by AD and nonaxial diffusivities when computing ADC as their sum. Nevertheless, in the literature, NAWM ADC increase in patients with IDHwt has been related to vasogenic edema and tumor infiltration–related tissue damage.18,29,30 Our results for IDHmut matched those in a recent study that showed lower NAWM FA and higher NAWM ADC and RD in IDHwt versus IDHmut,21 but a decrease in AD reported therein contradicts our observations. This issue potentially stems from using the analysis variable FA in the skeletonization algorithm31 for NAWM masking in Jütten et al21 versus our comprehensive nonselective NAWM masking.
Taking the AD and RD are markers for axonal integrity,32 in agreement with the findings of this study, a decrease in AD and FA and an increase in RD after radiation therapy were reported as resulting from radiation–related NAWM damage.33 A recent study found lower FA, and AD as well as higher ADC and RD in regions with high tumor infiltration.34 Furthermore, histopathologic studies also agree with higher ADC and lower FA of more aggressive genotypes, suggesting tumor infiltration–related damage with increasing infiltration.35,36 This also agrees with the observation where lower FA and RA for TERTonly indicate a tendency for molecular-motion isotropy in the WB-NAWM. However, pinpointing the microstructural changes behind the observations reported herein, especially in TERT subgroups, requires further histopathologic investigations.
In the IDHonly group, mostly negatively valued ΔAD distribution showed that AD tends to be larger on the ipsilateral side, arguing that the deformation/pressure caused by the tumor might be pushing the molecular motion in the direction of the major axis of the microstructure. In contrast, in the DN subgroup, the contralateral side tended to have larger AD, accordingly, ΔAD differentiates between only DN and IDHonly. Without notable physical deformation in the contralateral side, the causes behind major axis directional preference in groups with IDHwt are an open problem.
The interhemispheric observations, especially in aggressive genotypes such as IDHwt and TERTmut, including TERTonly, suggest that molecular motion was more isotropic in the ipsilateral hemisphere, potentially indicating loss of microstructural integrity therein. This suggestion agrees with findings in previous studies of decreasing microstructural organization in distal NAWM near the tumor.27 The observation in more aggressive genotypes may link the increased NAWM damage to higher-grade tumors where IDHwt and TERTmut genotypes are more prevalent.22
This investigation was limited by a construct of its methodology. Summarizing the properties of large ROIs such as the WB or the hemispheres with a single number, ie, the mean, is suitable for statistical analysis but limits their rich information content. Differing distributions from different patients might have the same mean, which raises the concern of hampering classification and thereby genotype prediction when the ROI means are used as features. By contrast, the full properties of distributions might better characterize microstructural phenotypes associated with the mutations, resulting in more accurate prediction thereof.
CONCLUSIONS
For NAWM, statistical analysis indicated that axial and nonaxial diffusivities, anisotropy indices, and interhemispheric differences proved to be the most associating variables for subgroups of IDH and TERT mutations. Additionally, the most basic classification methodology, ie, thresholding, provided optimistic classification performance despite its shortcoming as an one dimensional decision criterion. In the future, full distributions of the DTI variables from the WB, hemispheres, and their interhemispheric differences should be analyzed with machine learning methods for fully taking advantage of their richer information content residing in high-dimensional data spaces.
Acknowledgments
Hande Halilibrahimoğlu extends her special thanks to Seher Aydınlar and Mehmet Ali Aydınlar for their support in her research efforts while an undergraduate. The authors extend their deepest gratitude to the patients who consented to sharing their data for this effort against a complex and devastating disease.
Footnotes
The Scientific and Technological Research Council of Türkiye (TUBITAK) funded this work [216S432].
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received October 29, 2022.
- Accepted after revision March 21, 2023.
- © 2023 by American Journal of Neuroradiology
















