Abstract
BACKGROUND AND PURPOSE: Fractures with “vertebra plana” morphology are characterized by severe vertebral body collapse and segmental kyphosis; there is no established treatment standard for these fractures. Vertebroplasty and balloon kyphoplasty might represent an undertreatment, but surgical stabilization is challenging in an often elderly osteoporotic population. This study assessed the feasibility, clinical outcome, and radiologic outcome of the stent screw–assisted internal fixation technique using a percutaneous implant of vertebral body stents and cement-augmented pedicle screws in patients with non-neoplastic vertebra plana fractures.
MATERIALS AND METHODS: Thirty-seven consecutive patients with vertebra plana fractures were treated with the stent screw–assisted internal fixation technique. Vertebral body height, local and vertebral kyphotic angles, outcome scales (numeric rating scale and the Patient’s Global Impression of Change), and complications were assessed. Imaging and clinical follow-up were obtained at 1 and 6 months postprocedure.
RESULTS: Median vertebral body height restoration was 7 mm (+74%), 9 mm (+150%), and 3 mm (+17%) at the anterior wall, middle body, and posterior wall, respectively. Median local and vertebral kyphotic angles correction was 8° and 10° and was maintained through the 6-month follow-up. The median numeric rating scale score improved from 8/10 preprocedure to 3/10 at 1 and 6 months (P < .001). No procedural complications occurred.
CONCLUSIONS: The stent screw–assisted internal fixation technique was effective in obtaining height restoration, kyphosis correction, and pain relief in patients with severe vertebral collapse.
ABBREVIATIONS:
- ant
- anterior
- BKP
- balloon kyphoplasty
- IQR
- interquartile range
- LKA
- local kyphotic angle
- mid
- middle
- NRS
- numeric rating scale
- PGIC
- Patient’s Global Impression of Change
- post
- posterior
- SAIF
- stent screw–assisted internal fixation
- VB
- vertebral body
- VBH
- vertebral body height
- VKA
- vertebral kyphotic angle
- VP
- vertebra plana
- VBS
- Vertebral Body Stenting System
- VCF
- vertebral compression fracture
Painful osteoporotic vertebral compression fractures (VCFs) are commonly treated with traditional vertebral augmentation techniques, particularly vertebroplasty and balloon kyphoplasty (BKP), which reinforce the anterior column, arrest wedge deformity, and palliate pain. The VCFs characterized by severe vertebral body (VB) collapse (>70% VB height loss) are generally termed “vertebra plana” (VP)1 and demonstrate extreme osseous structural loss and resorption with anterior and middle column injury. Furthermore, they may present with intravertebral pseudoarthrosis (also termed cleft or Kümmel disease), posterior wall retropulsion, and pediculo-somatic junction fractures. The accompanying kyphosis can limit breathing2 and activities of daily living and is likely associated with an increased mortality risk.3
Ideally, treatment of these fractures should stabilize, restore height, correct sagittal spinal alignment, correct kyphotic deformity, and achieve pain relief. Although standard augmentation techniques are effective in achieving pain palliation,1,4⇓-6 they do not address middle column and pediculo-somatic junction fractures.7 Furthermore, secondary loss of stability has been reported at follow-up in VP cases treated with augmentation.8,9 At the same time, these fractures often affect elderly and fragile patients, making surgical stabilization problematic10,11 because stand-alone posterior fixation techniques carry high risk of failure in conditions of poor bone quality,12,13 while anterior or circumferential approaches are associated with higher intraoperative blood loss and perioperative complications.14 Thus, there is no standard treatment for these challenging VCFs.1,15
The stent screw–assisted internal fixation (SAIF) technique includes percutaneous insertion and balloon-expansion of 2 vertebral body stents (Vertebral Body Stenting System [VBS]; DePuy Synthes–Johnson & Johnson), followed by placement of cannulated and fenestrated pedicular screws (Injection pin, 2B1, Milan, Italy) in the lumen of the stents and cement augmentation through the screws. SAIF is currently being used for the treatment of severe osteoporotic and neoplastic fractures in 5 international centers.16⇓-18
The purpose of this study was to assess the feasibility and safety of performing SAIF in this cohort of patients with non-neoplastic VP. In addition, height restoration and kyphosis correction of the target vertebrae and the clinical outcome in terms of pain relief were studied.
MATERIALS AND METHODS
Patient Selection
This retrospective analysis included all thoracic and lumbar non-neoplastic VP treated with the stand-alone SAIF technique at a single institution between August 2017 and June 2020. Cases without comparable pre- and postoperative imaging (pre- and postoperative standing plain films, and/or pre- and postoperative CT/MR imaging) were excluded. The decision to treat with SAIF was made by a multidisciplinary group (composed of interventional neuroradiologists, spine surgeons, and pain specialists) for recent (<1 month) or nonhealed fractures causing persistent pain despite conservative treatment (≥5 on the numeric rating scale [NRS]; range, 0–10) or progressive collapse with kyphotic deformity. Nonhealed fractures were defined as osteoporotic fractures occurring >1 month earlier or at an unknown time with persistent pain and evidence of pseudoarthrosis (characterized by an intrasomatic cavity filled with gas or fluid and fracture mobility) and/or bone edema on MR imaging (STIR pulse sequence). Fractures were graded according to the AO Spine Spinal Section of the German Orthopedic and Trauma Society osteoporotic fracture classification system.19 Patients with neurologic deficits that required decompressive laminectomy and patients treated with SAIF combined with posterior instrumentation were excluded. All patients gave informed consent. The local ethics committee of Canton Ticino and EOC institutional review board approved the study.
SAIF Procedure
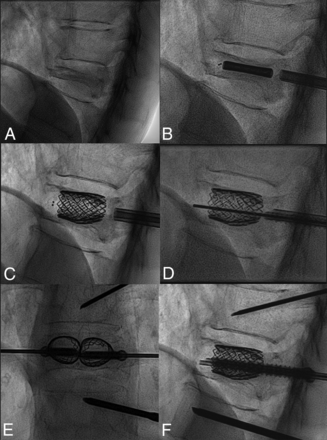
ll procedures were performed percutaneously with the patient under general anesthesia, with biplane fluoroscopic guidance using a previously described technique.16 Two VBSs were balloon-expanded in the VB with the intent to reduce local kyphosis. Transpedicular cannulated-fenestrated screws (Injection Pin 2B1; HealthManagement.org) were inserted over a Kirschner wire inside the VBS lumen and augmented with high-viscosity polymethylmethacrylate (Vertaplex HV; Stryker) under fluoroscopic control. Concomitant adjacent vertebroplasty was performed to treat milder VCFs (non-VP fracture: ie, with a minor degree of collapse) or with prophylactic intent when deemed appropriate per institutional protocol.20 The main procedural steps are summarized in Fig 1.
A, Procedural steps of the SAIF technique. Preprocedural lateral view of a T11 VP fracture. B, Balloon-mounted vertebral body stent insertion in the vertebral body. C, Balloon expansion of the stents. D, Access trocars are exchanged with transpedicular, cannulated-fenestrated screws over a Kirschner wire. Anterior-posterior and lateral views (E and F) obtained before cement injection through the screws.
Clinical and Imaging Assessment and Follow-up
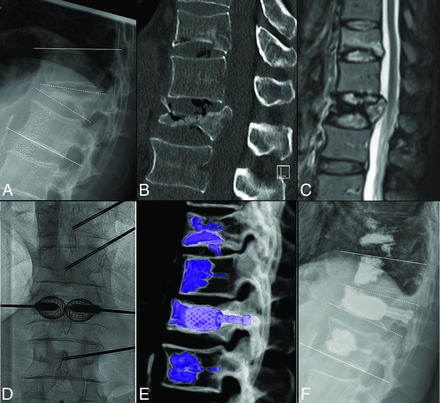
Intraprocedural and postprocedural complications were recorded according to the Clavien-Dindo classification.21 In patients with pre- and postoperative cross-sectional imaging (CT and/or MR imaging), the VB height (VBH) was measured on midsagittal images at the anterior wall (ant-VBH), middle body (mid-VBH), and posterior wall (post-VBH) (Fig 2). The percentage of VBH gain was calculated as the ratio of gained height/preprocedural height. In patients with standing x-rays, the local kyphotic angle (LKA, kyphotic angle of the 2 adjacent vertebral bodies) and the vertebral kyphotic angle (VKA, kyphotic angle of the fractured vertebral body)22 were measured pre- and postprocedure (Fig 2) and at follow-up. The percentage of kyphotic correction was calculated as the ratio of gained height/preprocedural height. All radiologic measurements were performed by 2 fellowship-trained neuroradiologists with 4 and 3 years of experience, respectively, not involved in the procedures.
A, Standing plain film shows a L1 VP with kyphotic angulation. White lines along the endplates of T12 and L2 indicate the LKA, while the dashed white lines along the L1 endplates indicate the VKA. B, Sagittal CT shows a pseudoarthrosis with a gas cleft in L1 and increased vertebral body height in supine decubitus positioning, in keeping with a mobile fracture. An additional fracture of T11 was treated with vertebroplasty. Sagittal fat-suppressed T2WI (C) shows posterior wall retropulsion and central canal stenosis without cord compression and an additional milder fracture at T11. Anterior-posterior intraprocedural fluoroscopic image (D) demonstrates SAIF implants, with pedicular screws inserted in the expanded stents before cement injection. Volume-rendering postprocedure CT (E) shows the SAIF treatment of L1 and vertebral augmentation at T11, T12, and L2. Postprocedural standing plain film (F) shows reduction of the LKA from 28° to 16° and of the VKA from 30° to 11°.
Preprocedural imaging was assessed to detect a cleft with pseudoarthrosis on CT/MR imaging and/or fracture mobility; fractures were categorized as mobile when VBH on standing views was reduced compared with supine views (Fig 2).
Clinical follow-up was performed at 1 and 6 months postoperatively and included the NRS (range, 0–10) and the Patient’s Global Impression of Change scale (PGIC; range, 1 = extremely worse, 4 = unchanged, to 7 extremely better).23 Kyphotic angles, mobilization of the implants, and refractures of the treated segment were assessed with standing x-rays.
Statistical Analysis
Analyses used SPSS, Version 20.0.0 (IBM). Descriptive statistics for demographic and clinical data were expressed as median with interquartile range (IQR). Differences in VBH, LKA, VKA, and NRS scores before and after treatment were tested by the Wilcoxon matched-pairs test; comparison between mobile and nonmobile fracture groups was tested by the Wilcoxon unpaired test. A P value < .05 was considered statistically significant.
RESULTS
Patients, Procedures, and Safety
The consecutive series of patients with vertebra plana treated with SAIF consisted of 42 cases; 5 were excluded due to lack of comparable pre- and postprocedure imaging. We, thus, included 37 SAIF procedures performed in 37 patients (11:26 male/female ratio; mean age, 81.6 years; range, 65–98 years). Between T3 and L4, 19/37 (51.3%) thoracic and 18/37 (48.6%) lumbar (overall, 27/37) (73%) fractures were located at the thoracolumbar junction (T10–L2). Thirty-four of 37 fractures were classified as osteoporotic fracture 4; three/37 fractures were classified as osteoporotic fracture 5 because of spinous process fracture (1/37) or mild posterior ligamentous complex lesions/edema (2/37) for which surgical instrumentation was withheld on the basis of the multidisciplinary spine care group recommendations. In 35/37 patients (94.6%), prophylactic vertebroplasty of adjacent levels was also performed (2/35 at the vertebra above, 33/35 above and below) at the operator’s choice per institutional protocol. All procedures were successfully completed without symptomatic cement leakage at the index level or clinical or technical complications.
Follow-up
Follow-up data with imaging and the patients’ outcome scales were available for 32/37 (86.5%; among them, 30 patients had comparable pre- and postprocedure CT/MR imaging and 29 patients had pre- and postprocedure standing x-rays) patients at 1 month and for 28/37 (75.7%) at 6 months. The remaining patients were contacted by a nurse on the phone to ascertain that no specific spinal problems had occurred, but in the absence of imaging and formal clinical data, those patients were not included in this analysis.
Radiologic Outcome
The Table summarizes the results.
Radiologic outcome: median measurements of anterior, middle, and posterior VBH, LKA, and VKA pre- and postoperatively (with IQR), for all fractures, mobile and nonmobile fracture groups
VBH.
Pre- and postoperative cross-sectional studies (CT or MR imaging) were available in 30/37 (81.1%) patients. In this group, the median ant-VBH, mid-VBH, and post-VBH were 9.5 mm (IQR = 8.0–13.0 mm), 6 mm (IQR = 5.0–7.75 mm), and 17.5 mm (IQR = 16.0–19.0 mm), respectively, preprocedure, and 17 mm (IQR = 15.0–19.25 mm), 15.5 mm (IQR = 13.0–17.25 mm), and 20 mm (IQR = 18.0–22.0 mm) postprocedure; the median height gain was 7 mm at the ant-VBH (+74%; range, 2–15 mm), 9 mm at the mid-VBH (+150%; range, 4–13 mm), and 3 mm at the post-VBH (+17%; range, 0–7 mm). All differences were statistically significant (P < .001).
LKA and VKA.
Pre- and postoperative standing x-rays were available for 29/37 (78.4%) patients.
In this group, the median LKA was 25° preoperatively (IQR = 12.0°–29.0°), and 14° postoperatively (IQR = 6.0°–22.0°). The median gain was 8° (range, 0°–19°) and was statistically significant (P < .001).
The median VKA was 21° preoperatively (IQR = 12.0°–27.°0) and 9.0° postoperatively (IQR = 5.5°–12.0°). The median gain was 10° (range, 1°–23°) and was statistically significant (P < .001).
In the patients with 6 months’ follow-up (28/37), the median LKA and VKA gains were substantially maintained, respectively, at 7° and 9°.
Mobile and Nonmobile Fractures.
Among 29 patients with preoperative standing x-rays available, a mobile fracture was present in 19 (65.5%) patients.
In this group, the median VBH gain was 7.0 mm (IQR = 5.5–8.5 mm) at the ant-VBH, 9 mm (IQR = 6.5–11.0 mm) at the mid-VBH, and 3 mm (IQR = 2.0–3.5 mm) at the post-VBH; the median correction of VKA and LKA was 11° (IQR = 5.0°–17.0°) and 8° (IQR = 7.0°–12.5°), respectively.
In patients with a nonmobile fracture (10/29), the median VBH gain was 8 mm (IQR = 7.0–9.75 mm) at the ant-VBH, 11 mm (IQR = 9.5–11.75 mm) at the mid-VBH, and 4.5 mm (IQR = 2.5–5.75 mm) at post-VBH; the median correction of VKA and LKA was 9.5° (IQR = 5.5°–15.5°) and 4.5° (IQR = 3.25°–7.75°), respectively.
The VBH and VKA corrections did not show significant differences between mobile and nonmobile fractures, while the LKA gain appeared greater in the mobile group, compared with nonmobile group, without reaching statistical significance (P = .07).
Refractures during Follow-up
No refracture occurred. No salvage surgery or new procedure was necessary at the index level during the available follow-up.
Pain Assessment
The median preoperative NRS pain score was 8 (range, 5–10; IQR = 7.0–9.0), while it was 3 (range, 0–8; IQR = 2.0–5.0) after 1 month and 3 (range, 0–7; IQR = 2.0–4.0) after 6 months. The differences were significant (P < .001). The median PGIC score was 6 (corresponding to “much better”) after 1 month (range, 4–7; IQR = 5.0–7.0) and remained 6 after 6 months (range, 3–7; IQR = 6.0–7.0). No significant difference was observed between mobile and nonmobile fractures in patients’ outcome scales (P = .35).
DISCUSSION
In this osteoporotic plana series, the SAIF technique was both feasible and safe. SAIF resulted in vertebral height restoration, kyphosis correction, and pain palliation. These results were sustained at 6 months’ follow-up.
Vertebral compression fractures with VP morphology are considered severe fractures,24,25 and surgical stabilization is generally recommended to restore segmental stability, allow early mobilization, and avoid pseudoarthrosis.10,26⇓-28 Kyphosis correction is important because kyphotic deformity is an independent risk factor for breathing difficulties and pulmonary complications, increasing morbidity and mortality.29,30
Open surgical treatment is typically recommended, including anterior instrumentation to reconstruct the anterior spinal column.14 However, these approaches may result in implant failure due to high strain31,32 and anterior or anterolateral approaches carrying higher risks of blood loss and respiratory complications in elderly and fragile patients.33
Vertebroplasty or BKP is less invasive and might provide pain relief but may represent an undertreatment for these severe fractures.4,6,8,34,35
Most published reports on the treatment of vertebral compression by BKP alone measured the postprocedure improvement of the VKA,36 but this measure might not translate into an effective segmental kyphosis correction.1,37 BKP might also be limited in effective kyphosis correction by the deflation effect before cement placement.38,39 The use of third-generation, rigid, intrasomatic distraction devices, such as SpineJack (Stryker) has been reported as a potential minimally invasive transpedicular replacement of expandable cages and, combined with posterior instrumentation, has been reported as a possible solution to treat VP fractures.27
The SAIF technique applies a treatment rationale that is well-suited to patients with severe vertebral collapse. The rigid stents obtain and maintain predictable fracture reduction, avoid deflation effect, and create room for cement, thus reducing the risk of leakage. The metallic mesh of the VBS scaffolds the vertebral body from within, offering ample cross-sectional support for the disc endplates. Cement injection then solidifies the structure and support. Percutaneous pedicle screws anchor the VBS-cement complex to the posterior elements. In addition, the screws offer osteosynthesis for pedicular fractures and act to transfer the spinal load to the neural arch, unloading the middle column.16 The “reinforced concrete” construct rebuilds the VB, offering a 360° nonfusion internal stabilization of the vertebra (Figs 3 and 4). Two biomechanical studies provide support for this approach in both neoplastic and osteoporotic models.7,40
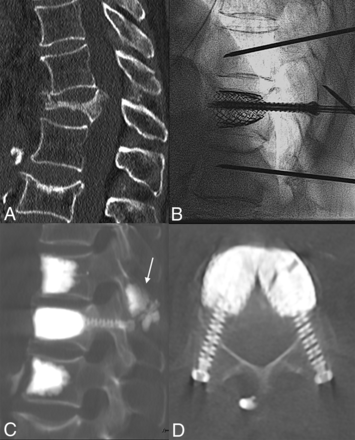
Sagittal CT (A) shows a T12 VP, with segmental kyphosis and a T11 spinous process fracture. Intraprocedural fluoroscopic lateral view (B) shows fracture reduction by the SAIF technique before cement augmentation. Postprocedural sagittal (C) and axial (D) CT images show the final results obtained with the SAIF construct. There is cement augmentation of the T11 spinous process fracture (arrow), which was particularly tender at palpation, and the prophylactic augmentation of the adjacent levels.
Standing plain film (A) and sagittal CT (B) show a T12 VP with pseudoarthrosis, gas cleft, and fracture mobility. Lateral intraprocedural fluoroscopic images before (C) and after (D) stent expansion with consequent fracture reduction. Postoperative standing plain film (E) demonstrates T12 height restoration and kyphosis correction, stable at 6 months’ follow-up (F). Axial CT (F) at the T12 level shows the stent-cement complex reconstructing the vertebral body and the transpedicular screws cemented inside the stents acting as “anchors” to the posterior elements.
In this series, SAIF obtained high degrees of VB height restoration, and VBH gain was much higher than previously reported with BKP.1 Yokoyama et al,41 using BKP, obtained 3.6-, 2.0-, and 0.5-mm VBH gain at the anterior, middle, and posterior VB, respectively. By means of the same measurements, SAIF obtained a median gain of 7.0, 9.0, and 3.0 mm.
LKA on standing plain radiographs was used to assess kyphosis correction. LKA has been demonstrated to be a valid and reliable measure of thoracic kyphosis in patients with osteoporosis, in addition to VKA.37 The LKA correction is usually less than the VKA correction because it is also influenced by the adjacent disc height loss but more reliably assesses the real effect of the treatment on segmental kyphosis (Fig 2).
In the present series, the LKA and VKA median correction was 8° and 10°, respectively, outperforming the previously reported results achieved with BKP, in which the LKA correction ranged between 1.94° and 6.5° and the VKA correction ranged between 4.2° and 7.3°.37,42⇓-44 Diel et al45 reported an average correction of LKA of 4.2° using VBS. Even when surgical posterior fixation was combined with vertebral augmentation, the LKA correction ranged between 5° and 9° in 3 studies.11 A recent prospective study reported an LKA correction of 9° at 1-year follow-up, obtained with augmentation, posterior instrumentation, and arthrodesis, followed by a plastic thoracolumbar orthosis to be worn for 6 months postsurgery.26 SAIF results on VBH and kyphosis correction were comparable with those obtained with 360° surgical approaches, but with a reduced complication rate.31
At 6-month follow-up, the achieved kyphosis correction was substantially stable, with an average loss of correction of only 1° at 6 months. In keeping with the previously reported results of Becker et al1 and Yokoyama et al,41 the LKA gain tended to be greater in the mobile fracture group, though the difference did not reach statistical significance, likely due to small numbers in the nonmobile group. Nevertheless, significant VBH and VKA correction was also obtained in nonmobile fractures. These results might be explained by the efficient distraction forces exerted by the stents and the avoidance of the deflation effect, with polymethylmethacrylate anchoring the entire complex to the vertebral body.
The axis of insertion of the vertebral body stent into the VB is of paramount importance to optimize craniocaudal distraction, fracture reduction, and height restoration. Pedicular access should, therefore, be adapted to optimize device placement inside the VB along an axis parallel to the anticipated alignment of the original prefracture endplates. The distraction performed perpendicular to this axis approximates the original prefracture shape of the VB and allows the device to achieve maximum expansion and fracture reduction.46 With the plana morphology, trocar access is usually through the lower half of the pedicle (Figs 3 and 4).
Prophylactic vertebroplasty of adjacent levels, the role of which remains controversial, was performed in 35/37 patients. High-quality evidence supporting improved patient outcome has not been confirmed.47 Of note, this study was performed in Switzerland where prophylactic augmentation is more commonly performed than in the United States.
Patients treated with SAIF had satisfactory pain relief and an overall subjective impression of improvement as measured by the NRS and the PGIC score, respectively. While pain relief has been similarly reported by standard augmentation techniques,48 the SAIF approach achieves greater improvement in kyphosis, potentially improving biomechanics, ambulation, and breathing function.
Patients requiring laminectomy were excluded from this series, but SAIF can be combined with decompression and posterior instrumentation when needed.
The main limitations of this study are the retrospective design and lack of a control group. Follow-up was generally limited to 6 months because our clinical practice does not require further medical visits for this fragile elderly population in the absence of persistent or new back pain. The single-center design limits its generalizability, and larger, multicenter prospective studies are warranted. Finally, the augmentation of the adjacent vertebral bodies (either to treat milder VCFs or for prophylactic intent) is an additional potential confounder with respect to pain relief. Of note, these specific vertebral body stents and percutaneous fenestrated screws lack US Food and Drug Administration approval, and these procedures have, thus far, all been performed in Europe.
CONCLUSIONS
This study suggests that SAIF can be performed safely in patients with severe vertebral collapse. SAIF was effective in obtaining vertebral body height restoration, kyphosis correction, and pain relief in this cohort with stability of these results at the 6-month follow-up assessment. Based on these preliminary results, SAIF could overcome some of the limitations of standard vertebral augmentation and present a minimally invasive option in patients with osteoporotic vertebra plana.
Footnotes
A. Cianfoni and R.L. Delfanti share first co-authorship.
M. Pileggi and J.A. Hirsch share last co-authorship.
Disclosure forms provided by the authors are available with the full text and PDF of this article at http://www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received October 17, 2021.
- Accepted after revision February 9, 2022.
- © 2022 by American Journal of Neuroradiology