Abstract
BACKGROUND AND PURPOSE: The presence of cerebral microbleeds has been associated with dementia and cognitive decline, although studies report conflicting results. Our aim was to determine the potential role of the presence and location of cerebral microbleeds in early stages of cognitive decline.
MATERIALS AND METHODS: Baseline 3T MR imaging examinations including SWI sequences of 328 cognitively intact community-dwelling controls and 72 subjects with mild cognitive impairment were analyzed with respect to the presence and distribution of cerebral microbleeds. A neuropsychological follow-up of controls was performed at 18 months post inclusion and identified cases with subtle cognitive deficits were referred to as controls with a deteriorating condition. Group differences in radiologic parameters were studied by using nonparametric tests, 1-way analysis of variance, and Spearman correlation coefficients.
RESULTS: Cerebral microbleed prevalence was similar in subjects with mild cognitive impairment and controls with stable and cognitively deteriorating conditions (25%–31.9%). In all diagnostic groups, lobar cerebral microbleeds were more common. They occurred in 20.1% of all cases compared with 6.5% of cases with deep cerebral microbleeds. None of the investigated variables (age, sex, microbleed number, location and depth, baseline Mini-Mental State Examination score, and the Fazekas score) were significantly associated with cognitive deterioration with the exception of education of >12 years showing a slight but significant protective effect (OR, 0.44; 95% CI, 0.22–0.92; P = .028). The Mini-Mental State Examination and the Buschke total score were correlated with neither the total number nor lobar-versus-deep location of cerebral microbleeds.
CONCLUSIONS: Cerebral microbleed presence, location, and severity are not related to the early stages of cognitive decline in advanced age.
ABBREVIATIONS:
- CDR
- Clinical Dementia Rating scale
- CMB
- cerebral microbleed
- dCON
- control with a deteriorating condition
- sCON
- control with a stable condition
- MCI
- mild cognitive impairment
- MMSE
- Mini-Mental State Examination
Cerebral microbleeds (CMBs) are small, round, or ovoid lesions of the cerebral parenchyma of low signal intensity on T2*-weighted and susceptibility-weighted sequences, with a maximal diameter of 5–10 mm,1 corresponding histologically to focal accumulations of hemosiderin-containing macrophages.2 They can be found in healthy subjects, their prevalence increasing with age,3⇓–5 but are more frequent in patients with hypertensive encephalopathy and cerebral amyloid angiopathy.1,2 Microbleeds have been considered markers of small-vessel disease and are strongly associated with white matter hyperintensities.3,6,7 Both hypertensive small-vessel disease and cerebral amyloid angiopathy contribute to the formation of lobar CMBs, while CMBs located in the basal ganglia or infratentorial brain regions are mainly associated with hypertensive vasculopathy.8⇓⇓–11
Their impact on cognition is still a matter of debate. Several studies supported a deleterious effect of CMBs, including increased prevalence in vascular dementia but also in Alzheimer disease,3,12⇓–14 associations with poorer cognitive function in cross-sectional studies of patients with dementia,15 lower Aβ42 levels in the CSF in Alzheimer disease and vascular dementia,16 and decreased frontal-executive performances at 5-year follow-up in patients with stroke.17 However, negative data were also reported with no or marginal impact of CMBs in early Alzheimer disease (for a review see van der Flier18 and Heringa et al19) and in subjects with subcortical vascular cognitive impairment20 and symptomatic small-vessel disease.21
Data on mild cognitive impairment (MCI) are even more ambiguous. This entity was initially used to denote a functionally nondisabling amnestic disorder, but its definition has been recently expanded to include any form of cognitive problem that may increase the risk of clinically overt dementia. Certain studies postulated that CMBs are significantly associated with both MCI and the risk of conversion to Alzheimer disease (for a review see Loitfelder et al8 and Lei et al22). Other authors reported a significant association between amyloid deposition and lobar CMB occurrence in patients with MCI but without any relationship between their presence and early cognitive decline.23 Similarly, substantial formation of lobar but not deep and infratentorial microbleeds was associated with worse cognition in the Rotterdam Scan Study.24 The latter is a population-based study on age-related changes on brain MR imaging. Cross-sectional analysis of 3979 individuals without dementia from this cohort revealed that subjects with higher numbers of lobar microbleeds performed worse in tests exploring various cognitive domains, even after adjustments for vascular risk factors and brain atrophy.24
Most of the previous studies concerned cross-sectional case-control comparisons and did not explore whether CMBs may predict very early phases of cognitive deterioration in healthy controls. Furthermore, the location of CMBs that may be associated with the disruption of brain networks was rarely taken into account.19 The current investigation is based on the assumption that if CMBs reflect structural damage, their location should have an impact on the corresponding function affected. To determine the potential role of CMBs in early stages of cognitive decline before MCI, we evaluated both the number and location of CMBs in a large sample of 328 community-dwelling healthy controls who were cognitively intact. Imaging was performed at baseline, and cognitive status was determined on the basis of extensive neuropsychological testing both at baseline and at 18-month follow-up. The results were compared with a group of 72 fully documented patients with MCI recruited in the same geographic area.
Materials and Methods
Participants
Participants were contacted via advertisements in local media to guarantee a community-based sample. After detailed information about the research was provided, telephone screening was performed with the following inclusion criteria: normal or corrected-to-normal visual acuity; no history of major medical disorders (neoplasm, cardiovascular disorders, infectious diseases), sustained head injury, or psychiatric or neurologic disorders; no alcohol or drug abuse; and no regular use of neuroleptics, antidepressants, mood stabilizers, anticonvulsant drugs, or psychostimulants. To control for the confounding effect of cerebrovascular diseases, we did not include patients with subtle cardiovascular symptoms, severe hypertension, and a history of stroke or transient ischemic episodes in the present study. Mild hypertension was present at baseline in 27% of the entire sample. The local ethics committee approved this prospective study, and all participants gave written informed consent before inclusion. The inclusion period for controls and those with MCI was from October 2010 to January 2011, when the present cohort was established in the context of a federally funded research project for identifying functional imaging and electroencephalography markers predicting subtle cognitive deficits in a community-dwelling sample of healthy controls. A relatively small number of patients with MCI were recruited as an additional control group.
Neuropsychological Assessment
All participants underwent extensive neuropsychological testing, as described in detail in the On-line Appendix. Briefly, all participants underwent neuropsychological testing and MR imaging at baseline. Participants classified as controls at baseline additionally underwent neuropsychological testing at 18-month follow-up. Those whose cognitive scores remained unchanged were classified as controls with a stable condition (sCON). Those whose performance at follow-up was at least 0.5 SDs lower compared with the first evaluation on at least 2 cognitive tests were classified as controls with a deteriorating condition (dCON). All individuals were also evaluated with the Clinical Dementia Rating scale (CDR).25 Only those with a CDR score of 0 and scores within 1.5 SDs of the age-appropriate mean in all other tests were included in the control group. In agreement with the Petersen criteria,26 participants having a CDR score of 0.5 but no dementia and a score exceeding 1.5 SDs below the age-appropriate mean in any of the above tests were confirmed as to their MCI status.
Two neuropsychologists clinically assessed all individuals independently with high interrater agreement (κ = 0.92). The final classification of sCON versus dCON was made by a trained neuropsychologist, who took into account both the neuropsychological test results and overall clinical assessment.27
MR Imaging
MR imaging was performed with a routine 3T scanner (Magnetom Trio; Siemens, Erlangen, Germany) and included a standard susceptibility-weighted sequence (matrix, 192 × 256 × 128; voxel size, 0.98 × 0.98 × 1.1 mm; TE/TR, 20/28 ms; number of signals acquired, 1; flip angle, 15°; parallel imaging factor, 2; acquisition time, 6 minutes 1 second). In addition, standard DTI, T2-weighted, T1-weighted, and fluid-attenuated inversion recovery sequences were performed and analyzed to exclude anomalies such as ischemic lesions, parenchymal macrobleeds, extra-axial hematomas, or space-occupying lesions.
Image Analysis
Cerebral microbleeds were defined as focal areas (<10 mm) of very low signal intensity. Two independent readers (1 board-certified neuroradiologist and 1 trained neuropsychologist with 7 and 3 years of experience, respectively) analyzed SWI to define the presence, number, and location of CMBs. In cases of discordant findings, a senior third reader (a board-certified neuroradiologist with 16 years of experience) reviewed the images and determined the final rating. CMB “mimics” such as signal voids caused by vessels or basal ganglia calcification were excluded.
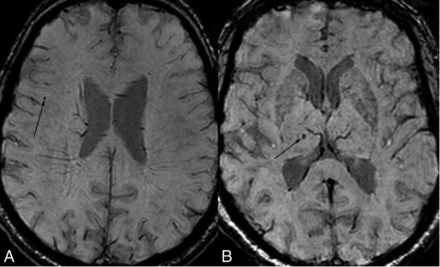
CMBs were classified according to 2 different categories (Figure): lobar versus deep (including the basal ganglia, thalamus, deep white matter, and infratentorial structures) and with further classification of lobar microbleeds according to the cerebral lobe involved (frontal, parietal, temporal, or occipital).
Cerebral microbleeds. Axial SWI of 2 subjects. A, Lobar CMB. Right inferior frontal CMB (arrow) close to the corticomedullary junction. B, Right thalamic CMB (arrow).
In addition to the analysis of CMBs, microvascular burden in the form of white matter lesions was analyzed on T2-/FLAIR-weighted images according to the established Fazekas scale.28
Statistical Analysis
χ2 tests, Kruskal-Wallis nonparametric tests, and 1-way ANOVA were used to compare binary/nominal, ordinal, and continuous Gaussian variables, respectively, among the 3 groups. The Cuzick nonparametric test for trend across ordered groups was used to compare the lobar distribution of microbleeds. A t test and the Mann-Whitney U test were applied to compare ordinal and continuous variables between 2 groups. Moreover, the number and location of CMBs were correlated with neuropsychological data at baseline by using the Spearman rank correlation. A multiple logistic regression model was built with sCON/dCON distinction as the dependent variable and age, sex, CMB location and depth, baseline Mini-Mental State Examination (MMSE) score, education, and Fazekas scale score as independent variables.
A multiple linear regression model was built to determine whether CMBs predict longitudinal changes in cognitive scores. Because cognitive performances at follow-up are expressed on different scales, which are often discrete, they cannot be linearly combined by adding the individual scores to a unique composite cognitive score. We converted all results to z scores; then, we summed the number of cognitive tests at follow-up with performances at least 0.5 SDs higher compared with the first evaluation, leading to the number of tests with improved performances (range, 0–14). Similarly, we summed the number of cognitive tests at follow-up with performances at least 0.5 SDs lower compared with the first evaluation, leading to the number of tests with decreased performances (range, 0–14). Finally, we computed the number of tests with improved minus the number of tests with decreased performances to obtain a continuous cognitive score and built a multiple linear regression model with this score as the dependent variable and age, sex, CMB location and depth, baseline MMSE score, education, and Fazekas scale score as independent variables.
All statistics were performed by using the STATA statistical software, Version 14.1 (StataCorp, College Station, Texas).
Results
Demographic Data
Demographic data of the cohort are shown in Table 1. There were no significant differences among the 3 groups (sCON, dCON, and MCI) regarding age (the mean age was 74 years) and education level. However, a significant difference was evident for sex with a male predominance in the MCI group (P < .001).
Demographic data for sCON, dCON, and MCI
Neuropsychological Data
Neuropsychological data are presented in Table 2. As expected, there were group differences at follow-up, with worse cognitive performances of dCON for the Shapes test (3 immediate recalls [P = .004] and delayed recall [P = .024]), Digit Symbol Coding (P < .001), and ideomotor transitive praxis (P = .008). The Shapes test assesses visual memory (immediate and delayed) via the reproduction of simple designs. Digit Symbol Coding (time-monitored copy of symbols) explores perceptual-motor speed mostly related to attention. Ideomotor transitive praxis refers to the ability to perform transitive movements demonstrating the use of tools.
Neuropsychological data of control subjects
Number of CMBs
Eleven subjects were excluded due to a presumed (incidental) diagnosis of amyloid angiopathy (based on the observation of multiple microbleeds at the corticomedullary junction in association with signs of superficial siderosis or sequelae of lobar hemorrhage) or hypertensive encephalopathy (based on the observation of microbleeds in association with extensive white matter signal anomalies and infarctions).
Most subjects had no CMBs: 75.0% of sCON, 72.7% of dCON, and 68.1% of MCI. Although the prevalence of at least 1 CMB increased from 25.0% in those with sCON to 28.3% in those with dCON and 31.9% in patients with MCI, group differences were not significant. There was no significant difference in the number of CMBs among the 3 groups (Table 3).
Microbleed numbers in the present seriesa
Location of CMBs
Lobar versus Deep.
Overall, lobar CMBs were more common and occurred in 20.1% of all cases compared with only 6.5% of cases with deep CMBs. There was no significant difference in the number of cases with lobar or deep CMBs among the 3 groups (Table 4).
Microbleed distributiona
Lobar Distribution.
There was no lobar predilection specific to 1 group. The highest prevalence of CMBs was found in the frontal lobe (11.3% of subjects having frontal lobe CMBs), followed by the occipital, parietal, and temporal lobes (6.5%, 5.3%, and 3.5%, respectively). There was no significant difference in the percentage of cases with CMBs among the 3 groups for any of these lobes, with the exception of the occipital lobe. The groups with more cognitive deficits had significantly fewer occipital lesions than those in the sCON group (P for trend = .0261) (Table 4).
Correlation of CMBs with Neurocognitive Testing
There was no significant correlation between the scores of the neurocognitive testing and the number or location of CMBs. For instance, the MMSE and the Buschke total score were correlated with neither the total number (Spearman ρ = −0.023, P = .653; ρ = −0.065; P = .213, respectively) nor the lobar-versus-deep location of CMBs (Spearman ρ = −0.013, P = .790; ρ = −0.064, P = .219, respectively). This finding was also the case for lobar distribution (data not shown).
A multiple logistic regression model (Table 5) showed that none of the investigated variables (age, sex, CMB number, location and depth, baseline MMSE score, and Fazekas score) were significantly associated with deterioration among controls as expressed by the dCON status, with the exception of education of >12 years showing a slight-but-significant protective effect (OR, 0.44; 95% CI, 0.22–0.92; P = .028).
Multivariate logistic regression to predict progression (sCON/dCON distinction)
Overall, 26% of the sample had a resulting decline in ≥2 cognitive tests when simultaneously taking into account both improved and decreased performances in the 14 cognitive tests. A multiple linear regression model (Table 6) predicting the number of cognitive tests (n = 14) with improved minus the number of tests with decreased performances by more than 0.5 SDs showed no effect of microbleeds and a protective effect of the MMSE score (coefficient, −0.35; 95% CI, −0.68–0.01; P = .042).
Multivariate linear regression to predict the number of cognitive tests (n = 14) that showed improvement minus the number of tests in which scores declined >0.5 SDs
Discussion
The current longitudinal, community-based study addresses the impact of CMBs in the very early stage of cognitive decline. We performed imaging at baseline in 328 elderly individuals with intact cognition at inclusion and determined very early cognitive decline based on neuropsychological follow-up at 18 months. Moreover, we compared these results with a group of 72 patients with fully documented MCI. We found no significant differences in the number or location of CMBs between controls with stable and deteriorating conditions and those with MCI. Moreover, there was no significant association between the number or location of CMBs and neuropsychological testing.
Prevalence of CMBs
Overall, the prevalence of CMBs in the present series varied from 25.0% in sCON to 28.3% in dCON and 31.9% in MCI. The trend toward increasing prevalence in MCI cases was not significant.
The prevalence in healthy controls (25.0%–28.3%) was higher than that reported in earlier studies.4,24,29 In particular, the Rotterdam Scan study that focused on elderly controls found a CMB prevalence of 15.3%.4,24 Differences in inclusion criteria and imaging techniques may be at the origin of these differences. Notably, our series included elderly individuals with a mean age of 74 years, clearly higher than that in previous reports. Because the prevalence of CMBs increases with age,3⇓–5 the higher age may partially explain the increased CMB prevalence observed here. In addition, technical differences among the studies, namely examinations at different field strengths (3T versus 1.5T) and with different sequences (SWI versus T2* gradient echo) may contribute to differing results. The present study used an SWI sequence obtained at 3T, while previous reports in controls used 1.5T machines and/or T2* sequences. It has been shown that SWI sequences detect more CMBs than 2D gradient-recalled echo sequences,30⇓–32 with an increase in detected lesions of 67% according to Nandigam et al.30
As in our study, the prevalence of CMBs was relatively similar in patients with MCI and controls (14% and 11%, respectively) in the cohort of Ayaz et al,33 including 28 healthy controls and 75 subjects with MCI examined at 1.5T with a SWI sequence. Other studies found an association between CMBs and low cognitive performance12 or cognitive decline.13 This variability may be due to differences in study design with varying cohort sizes and composition (eg, absence of a control group, varying exclusion criteria) and variable definitions of cognitive impairment. In the present study, the control group at baseline included only subjects who were cognitively intact confirmed by extensive neuropsychological testing. Moreover, subjects with a presumed (incidental) diagnosis of amyloid angiopathy or hypertensive encephalopathy were excluded to eliminate confounding effects.
CMB-Related Variables in the 3 Diagnostic Groups
The location of CMBs (ie, lobar versus deep and according to cerebral lobes) was not different among the 3 groups in our study with the exception of occipital lobe location. In the present series, subjects with MCI had a strikingly low prevalence of CMBs in the occipital cortex compared with sCON (P for trend = .0261). This latter group showed a similar CMB prevalence in frontal (comparable to MCI) and occipital cortices, excluding the idea of a preferential CMB formation in the visual cortex. Although unclear from a physiologic viewpoint, this finding further points to the dissociation between the formation of these lesions and cognitive decline.
In contrast, previous reports showed a strong association of CMB location with performance on cognitive tasks both in cross-sectional and longitudinal studies, though with conflicting results. Qiu et al12 found that deep hemispheric and infratentorial CMBs were associated with low performance, while in the Rotterdam Scan Study, strictly lobar CMB had the strongest impact on cognition.24 In a longitudinal study by Miwa et al,34 multiple CMBs or the presence of both deep and lobar CMBs (but not only strictly lobar CMBs) was associated with an increased risk for dementia, whereas Chiang et al35 found that lobar CMBs were associated with accelerated cognitive decline in their cohort. One could speculate that because CMBs reflect structural damage in a given region, their formation locally may affect the corresponding cognitive functions (eg, executive impairment in a frontal location). This was clearly not the case in the present series.
In fact, CMB presence and number did not correlate with neuropsychological variables in our cohort.
CMB-Based Prediction of Cognitive Decline in Healthy Elderly
In previous studies, greater or increasing numbers of CMBs with time were related to impaired cognitive functioning, in both cross-sectional12,24 and longitudinal analyses13,33⇓⇓–36 in different types of cohorts (eg, population-based or in a memory clinic setting). The nature of the associations between CMBs and cognitive performance was, however, variable and not necessarily independent. While the presence of CMBs was predictive of progression from MCI to dementia in the cohort of Kirsch et al,13 this association did not persist when adjusting for age. We found no association between cognitive decline at 18-month follow-up and CMB burden or location at baseline.
Several reasons may explain this clinicoradiologic dissociation. The low number of microbleeds in this community-based sample may prevent establishing valid correlations with clinical variables. Such correlations may become obvious at later time points at further follow-up; the absence of follow-up imaging constitutes one of the limitations of the present investigation. Similarly, the short clinical follow-up interval may have masked potential associations. Kirsch et al13 noted that during the 50-month follow-up of their study, only 5% of the subjects initially classified as healthy controls progressed to MCI or dementia. In the cohort of Miwa et al,34 8% of subjects developed dementia during a median follow-up of 7.5 years. Most of the prior studies did not investigate very early cognitive decline longitudinally. While 26% of controls in the present study showed deteriorating performance in an 18-month period, the investigated changes may have been too subtle, leading to the absence of an association with CMB observed here. In fact, we cannot formally exclude cognitive restoration possibly occurring at later time points in some of our subjects with dCON. One could speculate that a more prominent decline at later follow-up could allow identifying a cognitive impact of CMBs. However, this is unlikely because no difference in CMB prevalence was found between subjects with MCI with stable and deteriorating conditions in a prior study.36
Alternatively, structural damage reflected by isolated CMBs may not be important enough to impair clinically apparent locally associated functions, in contrast to the larger number of CMBs in vascular dementia and cerebral amyloid angiopathy (and Alzheimer disease).1,3,4
In contrast to CMB, there has been rising interest in the correlation of declining cognitive function and another possible marker of small (and large) vessel disease in dementia, cortical microinfarcts. This entity consists of lesions barely visible at conventional imaging but demonstrated at pathology and 7T MR imaging, with the lesions visible on imaging (especially at 3T) representing only a small fraction of the actual lesional burden.37 Future radiologic studies with new-generation MRIs may lead to better insight into the deleterious effects of widely disseminated microvascular changes in advanced age.
Conclusions
Ultimately, in this large extensively tested cohort of subjects with MCI and controls having undergone MR imaging at 3T with a SWI sequence, there was no group-level difference in microbleed prevalence or distribution or a correlation with neuropsychological test results.
Footnotes
Disclosures: Sven Haller—RELATED: Grant: Swiss National Foundation Grant SNF 3200B0-1161193 and SPUM 33CM30-124111.* *Money paid to the institution.
This study was partially funded by grants from the Swiss National Foundation (grant Nos. SNF 3200B0-1161193, SPUM 33CM30-124111).
Indicates open access to non-subscribers at www.ajnr.org
References
- Received March 15, 2016.
- Accepted after revision August 15, 2016.
- © 2017 by American Journal of Neuroradiology