Abstract
BACKGROUND AND PURPOSE: Oscillatory shear stress could not be directly measured in consideration of direction, although cerebrospinal fluid has repetitive movements synchronized with heartbeat. Our aim was to evaluate the important of oscillatory shear stress in the cerebral aqueduct and foramen magnum in idiopathic normal pressure hydrocephalus by comparing it with wall shear stress and the oscillatory shear index in patients with idiopathic normal pressure hydrocephalus.
MATERIALS AND METHODS: By means of the 4D flow application, oscillatory shear stress, wall shear stress, and the oscillatory shear index were measured in 41 patients with idiopathic normal pressure hydrocephalus, 23 with co-occurrence of idiopathic normal pressure hydrocephalus and Alzheimer-type dementia, and 9 age-matched controls. These shear stress parameters at the cerebral aqueduct were compared with apertures and stroke volumes at the foramen of Magendie and cerebral aqueduct.
RESULTS: Two wall shear stress magnitude peaks during a heartbeat were changed to periodic oscillation by converting oscillatory shear stress. The mean oscillatory shear stress amplitude and time-averaged wall shear stress values at the dorsal and ventral regions of the cerebral aqueduct in the idiopathic normal pressure hydrocephalus groups were significantly higher than those in controls. Furthermore, those at the ventral region of the cerebral aqueduct in the idiopathic normal pressure hydrocephalus group were also significantly higher than those in the co-occurrence of idiopathic normal pressure hydrocephalus with Alzheimer-type dementia group. The oscillatory shear stress amplitude at the dorsal region of the cerebral aqueduct was significantly associated with foramen of Magendie diameters, whereas it was strongly associated with the stroke volume at the upper end of the cerebral aqueduct rather than that at the foramen of Magendie.
CONCLUSIONS: Oscillatory shear stress, which reflects wall shear stress vector changes better than the conventional wall shear stress magnitude and the oscillatory shear index, can be directly measured on 4D flow MR imaging. Oscillatory shear stress at the cerebral aqueduct was considerably higher in patients with idiopathic normal pressure hydrocephalus.
ABBREVIATIONS:
- AD
- Alzheimer-type dementia
- iNPH
- idiopathic normal pressure hydrocephalus
- OSI
- oscillatory shear index
- OSS
- oscillatory shear stress
- TAWSS
- time-averaged wall shear stress
- WSS
- wall shear stress
Impaired CSF absorption might be caused by increased CSF volume in idiopathic normal pressure hydrocephalus (iNPH); however, its etiology has not been elucidated. Stroke volume at the cerebral aqueduct has been reported to be considerably increased in most patients with iNPH.1⇓⇓⇓⇓⇓⇓⇓-9 Our previous study on 4D flow MR imaging revealed that patients with iNPH had significantly increased reciprocating bidirectional CSF movements and flow-related bimodal high wall shear stress (WSS), a force vector produced by the fluid flow acting tangentially on the wall surfaces at the cerebral aqueduct in 1 cardiac cycle.10 Moreover, high time-averaged wall shear stress (TAWSS) due to bidirectional CSF movements at the cerebral aqueduct was significantly associated with z-axial expansion of the frontal horn of the lateral ventricles in iNPH. WSS is known to regulate vessel caliber and influences the development of vascular pathologies.11⇓⇓⇓⇓⇓⇓⇓⇓-20 Oscillating flow dynamics, specifically turbulent or disturbed flow, has been noticed in the pathogenesis of flow-mediated arterial dilation or atherosclerosis.11⇓⇓⇓⇓⇓⇓⇓⇓-20 Oscillating low WSS due to turbulent flow at the arterial bifurcation is associated with atherosclerotic plaque,11,16⇓-18 whereas chronic high WSS contributes to expansive or outward remodeling of vessels in response to a sustained increase in flow.19,20
In the CSF dynamics, the normal CSF movements generated by the directional beating of motile cilia on the ependymal cells lining the ventricular surface maintain a calm environment in the ventricles; however, increased reciprocating bidirectional CSF movements might induce the disruption of motile cilia and ependymal gliosis, which has been shown to be directly associated with ventriculomegaly.21⇓⇓⇓⇓-26 Therefore, we hypothesized whether oscillatory shear stress (OSS) due to bidirectional CSF movements at the cerebral aqueduct may also be associated with the size of the cerebral aqueduct and ventricles in iNPH. However, OSS could not be directly measured, which was different from the conventional WSS magnitude without direction information or the oscillatory shear index (OSI), indicating direction changes. Therefore, a novel method to directly measure OSS using 4D flow MR imaging was successfully developed. This study aimed to evaluate the significance of OSS at the cerebral aqueduct and foramen of Magendie in iNPH by comparing it with the conventional WSS magnitude and OSI. Furthermore, shear stress parameters were compared with apertures and stroke volumes at the foramen of Magendie and cerebral aqueduct among patients with iNPH only, patients with co-occurrence of iNPH and Alzheimer-type dementia (AD), and age-matched controls.
MATERIALS AND METHODS
The ethics committees for human research of Rakuwakai Otowa Hospital approved the study design and protocol (Nos. Rakuoto-Rin-17–041 and R2019-227). After obtaining written informed consent from patients or their relatives, the private information was anonymized in a linkable manner. Data collection, anonymization, image acquisition, and data-processing methods of 4D flow MR imaging were described in our previous study.10 In brief, 64 patients diagnosed with iNPH and 9 age-matched controls who underwent MR imaging examinations from January 28, 2017, to August 30, 2019, using a 3T MR imaging system (Magnetom Skyra; Siemens) were included in this study. All patients with iNPH had gait disturbance, cognitive impairment, and/or urinary incontinence, which improved after the CSF tap test, and ventricular dilation, an enlarged Sylvian fissure, and narrow sulci at the high convexity on conventional CT or MR imaging. A total of 23 patients with iNPH also had clinical AD based on the comprehensive assessment of their symptoms and findings on MR imaging and SPECT. Furthermore, 9 participants 60 years of age or older were recruited as controls because they had no symptoms of a short-stepped gait and/or cognitive impairment.
The time-resolved 3D velocity-encoding data were obtained from the 4D flow MR imaging sequence with 5 cm/s of velocity encoding and synchronizing the peripheral pulse rate measured from the finger (TR, 100 ms; TE, 9 ms; flip angle, 8°; FOV, 200 mm; matrix, 192 × 192; and voxel size, 1.0 × 1.0 × 1.3 mm). The image range was obtained in the midsagittal plane with a width of 30 mm (1.26 mm × 24 slices) from the bilateral foramina of Monro to the upper cervical subarachnoid spaces. All 4D flow analyses were conducted using the 4D flow application in an independent 3D volume analyzer workstation (Synapse 3D; Fujifillm Healthcare). To increase the accuracy of fine anatomic information of <1 mm, we used volumetric data on a T2-weighted 3D spin-echo sequence with sampling perfection with application-optimized contrasts by using different flip angle evolution (SPACE sequence; Siemens). Sequence parameters (TR, 2800 ms; TE, 286 ms; FOV, 230 mm; matrix, 192 × 192; and voxel size, 0.6 × 0.6 × 0.9 mm) were superimposed on 3D phase images using a trilinear interpolation algorithm, and a 3D polygon mesh of ventricles and subarachnoid spaces was created from this high-resolution volumetric data using a marching cubes algorithm (Online Supplemental Data). In all 4D flow analyses, stress parameters were measured after confirming the absence of phase aliasing or motion artifacts. The anterior-posterior diameters of the foramen of Magendie, the lower and upper ends of the cerebral aqueduct, and the midbrain were measured on the midsagittal plane of T2-weighted 3D SPACE MR imaging (Online Supplemental Data).
The 3D flow velocities (centimeters/second) and volumes (milliliters/second) of the reciprocating CSF movements through 12 ROIs were measured from the bilateral foramina of Monro to the lower end of the second cervical vertebra. The stroke volume was calculated by adding the absolute forward and backward CSF flow volumes, and reversed flow rate (%) by the absolute backward flow volume divided by the absolute forward flow volume. The surface mesh of CSF spaces was generated by increasing the image resolution and applying maximum smoothing, with an interpolation of a one-fourth interval and a decimation rate of 50%.
Shear Stress Parameters
The conventional WSS vector in the nth phase  (n) (N/m2) was calculated as
(n) (N/m2) was calculated as
 where Δz was the distance perpendicular to the wall, Δ
where Δz was the distance perpendicular to the wall, Δ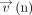 was the flow velocity vector in the nth phase parallel to the wall separate from Δz, and μ was the dynamic viscosity. In this study,
was the flow velocity vector in the nth phase parallel to the wall separate from Δz, and μ was the dynamic viscosity. In this study,  was set at 0.8 mm considering the resolution and average diameter of the cerebral aqueduct, and μ was set 1.0 × 0.001 (pascal × second) based on the CSF modeled as an incompressible Newtonian fluid with hydrodynamic characteristics of water.
was set at 0.8 mm considering the resolution and average diameter of the cerebral aqueduct, and μ was set 1.0 × 0.001 (pascal × second) based on the CSF modeled as an incompressible Newtonian fluid with hydrodynamic characteristics of water.
The WSS magnitude  was defined as an absolute value without direction information.
was defined as an absolute value without direction information.
To evaluate the cumulative effect of WSS vector during 1 heartbeat, we calculated the TAWSS vector 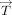 as
as
 where N was the total number of phases during 1 cardiac cycle and TAWSS magnitude
where N was the total number of phases during 1 cardiac cycle and TAWSS magnitude  was defined as a scalar quantity of the TAWSS vector.
was defined as a scalar quantity of the TAWSS vector.
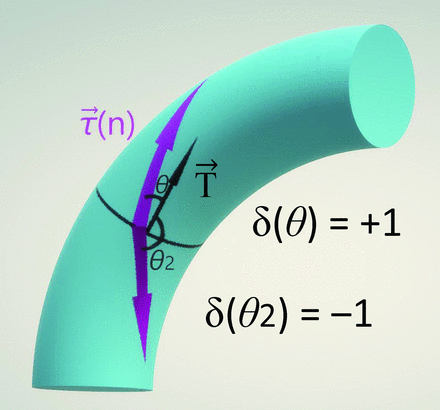
The angle between the nth phase direction of the WSS vector and the TAWSS vector as the principal axis was defined as θ(n), and the scale for converting θ(n) into a binary value of +1 or −1 was defined as  , calculated as
, calculated as
 where
where  = +1 indicates the same direction as
= +1 indicates the same direction as 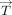 and
and  = −1 indicates a 180° opposite direction. As shown in Fig 1, the WSS magnitude multiplied by +1 or −1 was defined as OSS calculated as
= −1 indicates a 180° opposite direction. As shown in Fig 1, the WSS magnitude multiplied by +1 or −1 was defined as OSS calculated as
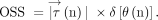
Schema explaining OSS: θ(n) is the angle between  and
and  in the nth phase
in the nth phase  (n)
(n)
 Where
Where 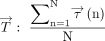 indicates a vector of TAWSS, and
indicates a vector of TAWSS, and  indicates a vector of WSS.
indicates a vector of WSS.
The OSS amplitude was calculated as the maximum minus the minimum OSS value.
OSI that monitors the direction changes of the WSS vector during 1 cardiac cycle was calculated as

The OSI ranges from 0 to 0.5, where 0 indicates a complete unidirectional flow and 0.5 indicates oscillatory flow only.
The WSS magnitude and OSI were automatically calculated per pixel and displayed on the 3D surface display using a scalable color map of the 4D flow application on Synapse 3D.
Data and codes used in this study are not available within the public domain because of the commercially available workstation.
The WSS magnitude, OSS, and OSI were automatically measured using 5 dotlike ROIs with the highest WSS magnitude at the ventral (ROI 1) and dorsal (ROI 2) regions of the foramen magnum and dorsal (ROI 3) and ventral (ROI 4) regions of the cerebral aqueduct and interpeduncular cistern (ROI 5).
Statistical Analysis
The Kruskal-Wallis rank sum test was used to compare the mean values [SD] for age and several MR imaging measurements among the 3 groups. In addition, the Wilcoxon rank sum test was used to compare continuous variables between patients with iNPH with co-occurrence of iNPH and AD or controls. The Fisher exact test was used to compare the 3 proportions. The Pearson correlation coefficient (r) and 95% CI were used to determine relationships between shear stress and morphologic parameters or stroke volume. Statistical significance was assumed at a P value < .05. All missing data points were treated as deficit data that did not affect other variables. Statistical analyses were performed using R statistical and computing software (Version 3.6.2; http://www.R-project.org).
RESULTS
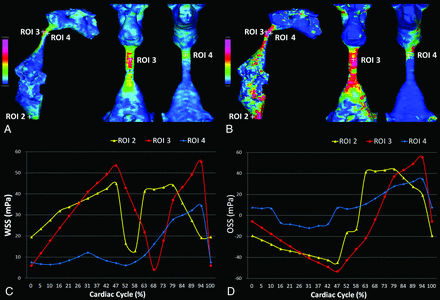
During 1 cardiac cycle, the CSF moved in the craniocaudal and caudocranial directions periodically through the cerebral aqueduct (Fig 2 and Online Supplemental Data). Bidirectional CSF movements at the dorsal region of the cerebral aqueduct were larger than those in the ventral region in patients with iNPH. Chronologic WSS magnitude changes in the dorsal region of the cerebral aqueduct were also larger than those in the ventral region due to bidirectional CSF movements (Fig 3 and Online Supplemental Data). The distribution of WSS magnitude (Fig 3A) was somewhat different from that of the OSI (Fig 3B). The bimodal high WSS magnitude in Fig 3C at the dorsal region of the foramen magnum (ROI 2) and cerebral aqueduct (ROI 3) was changed to the periodic oscillation by converting OSS (Fig 3D). In other words, 1 of the 2 WSS magnitude peaks during a heartbeat was converted to the negative OSS.
Oscillation of WSS due to bidirectional CSF flow through the cerebral aqueduct. The numbers 1, 2, and 3 demonstrate temporal changes of the reciprocating CSF motion during 1 cardiac cycle in a 77-year-old man diagnosed with iNPH. The colored path lines (lower figures) show the flow as a moving trajectory of the virtual particle through the middle part of the cerebral aqueduct in 1 heartbeat. The color and length of 3D streamlines (upper figures) indicate the flow velocity as pink for fast and blue for slow. The sampling interval of streamlines and path lines was set at 0.2 mm after the trilinear interpolation. The arrows indicate the WSS vector produced by the flow parallel to the wall surface. The size and direction of the arrows indicate the magnitude and direction of the WSS vector. Temporal changes of the WSS vector at the dorsal region (yellow arrows) were larger than those at the ventral region (white arrows).
Distributions and fluctuations of shear stress parameters per heartbeat in iNPH. The colored surfaces in the ventricles show the distribution of WSS magnitude (A) and OSI (B) in the same patient with iNPH, as shown in Fig 2. Red indicates high and blue indicates low. Line-graphs C and D show chronologic changes of WSS magnitude and OSS at the dorsal region of the foramen magnum (ROI 2, yellow) and dorsal (ROI 3, red) and ventral (ROI 4, blue) regions of the cerebral aqueduct in 1 cardiac cycle. Considering the shear stress direction, these changes were completely different.
A total of 73 participants comprising 41 patients diagnosed with iNPH only, 23 with co-occurrence of iNPH and AD, and 9 age-matched controls were included in this study (Table 1).
Clinical and morphologic characteristics of the study populationa
The difference in mean OSS amplitude, TAWSS, and OSI at the ventral (ROI 1) and dorsal (ROI 2) regions of the foramen magnum and interpeduncular cistern (ROI 5) was not significant among the 3 groups. Therefore, the amplitude, maximum, and minimum OSS values; TAWSS; and OSI at the dorsal (ROI 3) and ventral (ROI 4) regions of the cerebral aqueduct are shown in Table 2. The mean OSS amplitude at the dorsal and ventral regions of the cerebral aqueduct in the iNPH groups was approximately 3 times higher than that in the controls because the maximum OSS in the iNPH groups was twice as high and the minimum OSS was considerably lower. Remarkably, the mean amplitude and maximum OSS values at the ventral region of the cerebral aqueduct in the iNPH-only group were twice as high as those in the iNPH with AD group. The mean TAWSS magnitude at the dorsal region of the cerebral aqueduct in the iNPH-only group was also significantly higher than that in the controls. Furthermore, patients with iNPH-only had a significantly higher mean TAWSS magnitude at the ventral region of the cerebral aqueduct compared with those with iNPH with AD. The median and distribution of the OSS amplitude and TAWSS magnitude at the dorsal and ventral regions of the cerebral aqueduct among the 3 groups are shown as boxplots in Fig 4. Both the OSS amplitude and TAWSS magnitude at the dorsal region of the cerebral aqueduct in the iNPH groups were significantly higher than those in the controls, whereas the OSS amplitude at the ventral region of the cerebral aqueduct in the iNPH-only group was significantly higher than that in the iNPH with AD group and controls. The difference in OSI was not significant among the 3 groups.
Boxplots for the amplitude of OSS and TAWSS at the dorsal and ventral regions of the cerebral aqueduct among 3 groups. A, Distribution of the OSS amplitude at the dorsal region of the cerebral aqueduct. B, Distribution of the TAWSS at the dorsal region of the cerebral aqueduct. C, Distribution of the OSS amplitude at the ventral region of the cerebral aqueduct. D, Distribution of the TAWSS at the ventral region of the cerebral aqueduct.
Mean value [SD] of parameters at the cerebral aqueduct in the study populationa
The TAWSS magnitude was strongly associated with the amplitude and maximum and minimum OSS values at the dorsal (r = 0.89, 0.93, and −0.77) and ventral (r = 0.88, 0.93, and −0.73) regions of the cerebral aqueduct (Online Supplemental Data).
Relationships between Shear Stress Parameters and Anterior-Posterior Diameter or Stroke Volume
The amplitude and maximum and minimum OSS values and TAWSS magnitude at the dorsal region of the cerebral aqueduct were significantly associated with the anterior-posterior diameters of the foramen of Magendie and the lower end of the cerebral aqueduct (Table 3) and were strongly associated with stroke volume at the upper and lower ends of the cerebral aqueduct and foramen of Magendie, respectively (Table 4). The amplitude and maximum and minimum OSS values and TAWSS magnitude at the ventral region of the cerebral aqueduct were significantly-but-weakly associated with stroke volumes at the lower and upper ends of the cerebral aqueduct. However, OSI was not significantly associated with anterior-posterior diameters or stroke volumes at any locations.
Relationships with anterior-posterior diameter by the Pearson correlation coefficient (95% confidence intervals)
Relationships with stroke volume by the Pearson correlation coefficient (95% confidence intervals)
DISCUSSION
The reciprocating bidirectional CSF movements increase the bimodal WSS magnitude at the cerebral aqueduct during a cardiac cycle in iNPH. The conventional WSS magnitude was calculated as an absolute value of the WSS vector without the direction information by converting to a scalar quantity. However, the actual shear stress was produced by the bidirectional CSF flows from positive to negative values, not in a bimodal waveform. Therefore, a novel parameter, OSS with plus/minus directional information in addition to the WSS magnitude, was developed. On the basis of our literature review, this study is the first report that directly measures the OSS on 4D flow MR imaging. The maximum value of positive OSS drastically fluctuated to the minimum value of negative OSS during a heartbeat due to the reciprocating CSF movements in iNPH. Conversely, the maximum WSS magnitude value was either of the 2 peaks, whereas the minimum value was nearly zero consistently. However, the TAWSS magnitude was significantly associated with the OSS amplitude.
The distribution and magnitude of OSI were different from those of OSS or WSS magnitude. In contrast to steady flow conditions, pulsatile bidirectional flows cause large OSS fluctuations.
In this study, the OSS amplitude at the dorsal region of the cerebral aqueduct was significantly associated with both the foramen of Magendie diameters and stroke volumes at the upper and lower ends of the cerebral aqueduct and foramen of Magendie, respectively. Our previous study demonstrated that the stroke volume at the cerebral aqueduct had the strongest association with the foramen of Magendie diameter.10 These findings indicate that the dilation of the foramen of Magendie may be the first trigger for increased OSS amplitude at the dorsal region of the cerebral aqueduct due to increased aqueductal stroke volume. On the basis of study on oscillating flow dynamics,11⇓⇓⇓⇓⇓⇓⇓⇓-20 chronic high OSS at the cerebral aqueduct may be involved in the expansive remodeling of the aqueductal lumen diameter and may subsequently increase the pulsatile CSF inflow. However, this study has not yet proved this evidence.
Increased OSS amplitude may be associated with symptoms or pathologies of iNPH. We found that patients with iNPH-only had significantly higher OSS amplitude only at the ventral region of the cerebral aqueduct compared with those with co-occurrence of iNPH and AD. This finding was consistent with our previous result that patients with iNPH-only had not only smaller ventricular volume but also larger basal cistern and Sylvian fissure volumes than those with co-occurrence of iNPH and AD.27 AD is known as the most common comorbidity with iNPH due to CSF stagnation,28⇓⇓⇓-32 hindering the clearance of neurotoxic molecules such as amyloid-β or τ.33,34 Recently, pulsatile CSF inflow to the ventricular systems during sleep was reportedly associated with large, coupled low-frequency oscillations in the neuronal activity and hemodynamic oscillations.35 Decreased CSF pulsation may be associated with AD onset through the pathway in suppressed electrophysiologic slow waves that impair amyloid-β and τ excretion into the brain interstitial fluid and CSF during sleep.36⇓⇓-39 Therefore, direct OSS measurement in future studies will help determine whether CSF oscillations are associated with the AD onset.
This study had some limitations as described in our previous study.10 In particular, influences of respiration, wall motion, eddy currents, and background noise on the 4D flow MR imaging sequence were not assessed. Second, OSS and WSS magnitudes calculated by 4D flow MR imaging may be underestimated compared with the true values, though their distribution is considered reasonably accurate.40 Third, the AD comorbidity was not pathologically confirmed through brain biopsy, CSF biomarkers, or amyloid imaging in this study. Finally, we were unable to determine a causal relationship between the dilated cerebral aqueduct and increased OSS amplitude because this study had a cross-sectional design. Therefore, a causal relationship should be investigated in a prospective cohort study or basic research.
CONCLUSIONS
OSS as a novel parameter combining the conventional WSS magnitude with a positive or negative sign indicating the WSS direction can be quantitatively measured at any point on 4D flow MR imaging. Therefore, the OSS amplitude produced by reciprocating bidirectional CSF movements reflects WSS vector changes better than the conventional scalar quantities of the WSS magnitude and OSI. Quantitative OSS measurement may help elucidate the pathophysiologic mechanism of ventricular dilation in iNPH or symptom progression. OSS brings a new perspective to the study of slowly and intricately moving CSF in the complex shapes of the ventricles and the subarachnoid space.
Acknowledgments
We thank the patients and volunteers for their participation and the radiologists for their cooperation in the study. We also thank Enago (www.enago.com) for the English language review.
Footnotes
The funding sources for the study had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
This research received grants from the G-7 Scholarship Foundation for 1 year in 2020, the Taiju Life Social Welfare Foundation for 1 year in 2020, and the Fujifilm Corporation for 2 years since 2019.
Disclosures: Shigeki Yamada—RELATED: Grant: Fujifillm Corporation, Comments: This study received funding of 500,000/year × 2 years from Fujifillm Corporation in Japan. Our institute (Shiga University of Medical Science) has signed a research contract with Fujifillm Corporation for the joint development of 3D workstation applications*; UNRELATED: Grants/Grants Pending: G-7 Scholarship Foundation, Comments: research grant*; Payment for Lectures Including Service on Speakers Bureaus: Fujifilm Medical Systems, Integra Japan, and Daiichi Sankyo, Comments: Speakers honoraria. Masatsune Ishikawa—RELATED: Grant: Health and Labor Sciences Research Grants for the Research on Intractable Diseases, Ministry of Health, Labor and Welfare, Japan, Comments: 2017-Nanci-General-037. Kazuhiko Nozaki—UNRELATED: Grants/Grants Pending: Japan Agency for Medical Research and Development, grants from KAKENHI, grant from the Japan Society for the Promotion of Science*; Payment for Lectures Including Service on Speakers Bureaus: honoraria from Pfizer Japan, Daiichi Sankyo. *Money paid to the institution.
References
- Received May 22, 2020.
- Accepted after revision October 5, 2020.
- © 2021 by American Journal of Neuroradiology