Abstract
SUMMARY: The 3D edge-enhancing gradient-echo (EDGE) MR imaging sequence offers superior contrast-to-noise ratio in the detection of focal cortical dysplasia. EDGE could benefit from 7T MR imaging but also faces challenges such as image inhomogeneity and low acquisition efficiency. We propose an EDGE-MP2RAGE sequence that can provide both EDGE and T1-weighted contrast, simultaneously, improving data-acquisition efficiency. We demonstrate that with sequence optimization, EDGE images with sufficient uniformity and T1-weighted images with high gray-to-white matter contrast can be achieved.
ABBREVIATIONS:
- CNR
- contrast-to-noise ratio
- EDGE
- edge-enhancing gradient-echo
- FCD
- focal cortical dysplasia
- INV
- inversion imaging
Focal cortical dysplasia (FCD) is the most commonly resected epileptogenic lesion in children and the third most common lesion in adults. A recently described MR imaging sequence, 3D-edge-enhancing gradient-echo (3D EDGE), offers superior contrast-to-noise (CNR) in the detection of FCD.1,2 However, the sequence has inherently low SNR and would theoretically be advantageous at 7T. However, 7T MR imaging presents unique challenges such as severe transmit B1 field (B1+) inhomogeneity, which can greatly compromise image-contrast uniformity. Additionally, the longitudinal T1s of brain tissues are prolonged at 7T, which requires a longer time for the longitudinal magnetization to recover. For EDGE, the longitudinal magnetization has a substantial impact on overall image contrast and SNR. Due to the prolonged T1, a longer TR may be necessary to improve the SNR and image uniformity, though it decreases data-acquisition efficiency and results in longer scan times.
We describe a new strategy to acquire the EDGE contrast to improve the acquisition efficiency. Because high-resolution, 3D T1-weighted imaging, such as an MPRAGE, is typically needed in an epilepsy MR imaging protocol, simultaneous acquisition of both T1-weighted and EDGE images in a single sequence can improve data-acquisition efficiency while allowing a longer TR to improve image uniformity and SNR. This acquisition strategy is accomplished by integrating a second acquisition window into the EDGE sequence, resulting in an MP2RAGE sequence.3 In this study, we explore this strategy, EDGE-MP2RAGE, on clinical 7T MR imaging and show that it is capable of producing high-quality EDGE and T1-weighted images, which may be useful for clinical epilepsy imaging at 7T.
TECHNIQUE
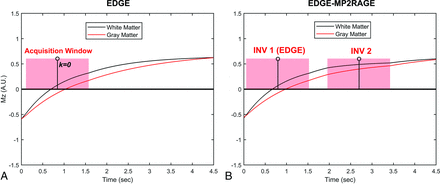
EDGE is a magnetization-prepared fast gradient-echo sequence with its TI set at the time when the GM and WM longitudinal magnetizations have comparable signal intensity but opposite polarity, therefore offering a signal-cancellation effect at their boundaries (Fig 1A).2 A long TR can lead to increased system idle time during which no data are acquired, increasing the overall scan time. Figure 1B demonstrates the proposed EDGE-MP2RAGE sequence, in which a second data-acquisition window with a different TI is added to the same TR following the EDGE acquisition. The 2 sets of raw inversion images (INV), INV 1 and 2, can be used to generate additional T1-weighted images similar to those in conventional MP2RAGE. Such an approach allows 2 image contrasts to be generated from the same TR, thereby improving data-acquisition efficiency while allowing a longer TR to be used to boost image SNR.
Examples of WM and GM longitudinal magnetizations (Mz) evolution in a repetition period (TR) for the EDGE (A) and EDGE-MP2RAGE (B) sequences. With EDGE contrast (A), the center of the data-acquisition window, which coincides with the k-space center (ie, k = 0), is placed when WM and GM have comparable signal intensity but opposite polarity. With EDGE-MP2RAGE (B), the first acquisition (ie, INV 1) is designed to provide the EDGE contrast. A.U. indicates arbitrary unit.
For a conventional MP2RAGE sequence, the TIs of the 2 acquisitions are typically optimized for the GM-WM CNR in the final T1-weighted images.3 With EDGE-MP2RAGE, however, sequence optimization is more complicated due to the requirement to adjust the INV1 to yield an EDGE contrast with sufficient SNR. Meanwhile, additional consideration is needed to improve the EDGE contrast uniformity across the brain, which can be compromised by the high B1+ inhomogeneity at 7T.
The proposed EDGE-MP2RAGE accounts for these challenges by optimizing the sequence parameters to accomplish 2 objectives simultaneously, ie, to provide INV1 with EDGE contrast of sufficient SNR and image uniformity and to provide calculated T1-weighted images of sufficient GM-WM CNR. To achieve these objectives, we performed numeric simulations based on Bloch equations to determine the optimal pulse sequence parameters. The signal intensities of GM and WM in the INV1, INV2, and T1-weighted images were calculated on the basis of Bloch simulations, assuming different combinations of sequence parameters (ie, TI, TR, flip angle, echo spacing, and acquisition bandwidth). From this analysis, a group of parameter combinations that allow comparable GM/WM signal intensities in INV1 across a range of B1+ inhomogeneity at 7T was determined.3 Finally, within this group, we selected the combination of parameters that were optimized simultaneously for both the SNR in the INV1 and the GM-WM CNR in the T1-weighted image.
The optimized sequence was then implemented on a clinical 7T Magnetom Terra MR imaging (Siemens) scanner with an 8Tx/32Rx head coil (Nova Medical) operating in single-transmit mode (TrueForm). Following an institutional review board–approved protocol, EDGE-MP2RAGE images were acquired on a healthy subject (a 17-year-old girl).
RESULTS
The Table shows the sequence parameters determined from the Bloch simulation analysis, which were designed assuming an isotropic resolution of 0.8 mm, giving a total scan time of 9 minutes. The full scan protocol can be found in the Online Supplemental Data.
EDGE-MP2RAGE sequence parameters
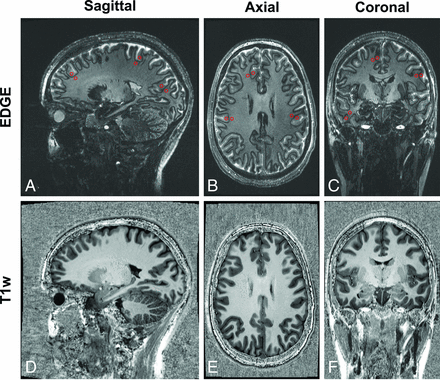
Examples of EDGE (INV1) images acquired using the proposed EDGE-MP2RAGE sequences are shown in Fig 2, together with the T1-weighted images. The INV1 (A–C) show that WM and GM have comparable signal intensities, consistent with the EDGE contrast. For quantitative analysis, the cortical WM and GM signal intensities were measured from the EDGE images using ROIs across the brain in 3 orthogonal planes. The results show that the mean WM and GM signal levels are nearly identical (78 [SD, 12] versus 77 [SD, 10], respectively), confirming the visual analysis. Figure 2 also shows that high-quality T1-weighted images with good GM-WM contrast can be generated from the same acquisition. The EDGE image SNR, calculated as previously described for a single EDGE acquisition, measures 59.1 compared with approximately 30 reported for 3T EDGE.4
Examples of EDGE (INV 1, A–C) images and T1-weighted (T1w) images (D–F) acquired with the EDGE-MP2RAGE reformatted into sagittal, axial, and coronal planes. The ROI (red circles in A–C) for WM and GM signal intensity measurements are also shown.
DISCUSSION
In this work, we developed and optimized a new EDGE-MP2RAGE sequence for use in MR imaging studies of epilepsy at 7T. Compared with lower fields, 7T allows higher-resolution imaging due to the high intrinsic SNR. However, due to the high B1+ inhomogeneity and longer tissue T1 values, it is challenging to implement the EDGE contrast at 7T. To improve SNR and contrast uniformity, one can use a longer TR; however, this substantially reduces data-acquisition efficiency. By means of the EDGE-MP2RAGE sequence, an additional T1-weighted contrast can be generated from the same acquisition, and the average time spent on acquiring each contrast is effectively reduced by 50%, making it feasible to be implemented in clinical examinations. As demonstrated in the results section, EDGE contrast with acceptable image uniformity can be achieved with this approach using carefully selected sequence parameters despite well-known high B1 inhomogeneity at 7T, and T1-weighted images with high GM-WM contrast can also be produced from the same acquisition. Note that the protocol described in this work is designed for 7T imaging and is not directly applicable to lower fields, such as 3T, because the longitudinal T1s of various brain tissues are different at other field strengths.
EDGE MR imaging has been shown to produce a substantial increase in CNR in the setting of FCD compared with traditional sequences such as MP2RAGE, FLAIR, and double inversion recovery.2 The added SNR from 7T allows higher image resolution (0.8 versus 1.0 mm) while still achieving nearly twice the SNR of 3T EDGE. Additionally, a 3D T1-weighted image is also a hallmark of epilepsy protocols, allowing detailed assessment of brain anatomy. At 3T, the application of 2 TIs to generate an MP2RAGE image has been shown to be superior to MPRAGE for evaluation of epilepsy.3 At 7T, the advantages of MP2RAGE over MPRAGE are even greater due primarily to the severe nonuniformities in the transmit field (B1+), which is reduced in MP2RAGE.5 Within our framework, we have shown how the sequence parameters can be simultaneously optimized for EDGE contrast as part of the first TI image, while also optimizing the resulting T1-weighted image (also known as UNI image) of MP2RAGE.
One limitation of this study, however, is that a direct technical comparison between the new sequence and the standard ones has not been made, and this could be the object of future works.
CONCLUSIONS
We describe an optimized protocol for simultaneous EDGE-MP2RAGE imaging at 7T and show that this protocol could be useful for imaging drug-resistant focal epilepsy.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
- Received August 15, 2022.
- Accepted after revision January 3, 2023.
- © 2023 by American Journal of Neuroradiology