Abstract
BACKGROUND AND PURPOSE: While contrast-enhanced MR imaging is the criterion standard in meningioma diagnosis and treatment response assessment, gallium 68Ga-DOTATATE PET/MR imaging has increasingly demonstrated utility in meningioma diagnosis and management. Integrating 68Ga-DOTATATE PET/MR imaging in postsurgical radiation planning reduces the planning target volume and organ-at-risk dose. However, 68Ga-DOTATATE PET/MR imaging is not widely implemented in clinical practice due to higher perceived costs. Our study analyzes the cost-effectiveness of 68Ga-DOTATATE PET/MR imaging for postresection radiation therapy planning in patients with intermediate-risk meningioma.
MATERIALS AND METHODS: We developed a decision-analytical model based on both recommended guidelines on meningioma management and our institutional experience. Markov models were implemented to estimate quality-adjusted life-years (QALY). Cost-effectiveness analyses with willingness-to-pay thresholds of $50,000/QALY and $100,000/QALY were performed from a societal perspective. Sensitivity analyses were conducted to validate the results. Model input values were based on published literature.
RESULTS: The cost-effectiveness results demonstrated that 68Ga-DOTATATE PET/MR imaging yields higher QALY (5.47 versus 5.05) at a higher cost ($404,260 versus $395,535) compared with MR imaging alone. The incremental cost-effectiveness ratio analysis determined that 68Ga-DOTATATE PET/MR imaging is cost-effective at a willingness to pay of $50,000/QALY and $100,000/QALY. Furthermore, sensitivity analyses showed that 68Ga-DOTATATE PET/MR imaging is cost-effective at $50,000/QALY ($100,000/QALY) for specificity and sensitivity values above 76% (58%) and 53% (44%), respectively.
CONCLUSIONS: 68Ga-DOTATATE PET/MR imaging as an adjunct imaging technique is cost-effective in postoperative treatment planning in patients with meningiomas. Most important, the model results show that the sensitivity and specificity cost-effective thresholds of 68Ga-DOTATATE PET/MR imaging could be attained in clinical practice.
ABBREVIATIONS:
- CTCAE
- Common Terminology Criteria for Adverse Events
- EBRT
- external beam radiotherapy
- GTR
- gross total resection
- PFS
- progression-free survival
- QALY
- quality-adjusted life-years
- RT
- radiotherapy
- SRS
- stereotactic radiosurgery
- SSTR2A
- somatostatin receptor 2A
- WHO
- World Health Organization
- WTP
- willingness to pay
Meningiomas are the most common primary benign brain neoplasms.1 Surgery is the primary management, particularly for larger or symptomatic tumors. The World Health Organization (WHO) classification stratifies patients on the basis of histopathologic features (number of mitotic figures per high-power field), and it is used in prognostication and postoperative radiation therapy planning. While most meningiomas are grade 1, approximately 15%–20% are grade 2 or 3, demonstrating more aggressive behavior and worse prognosis. Gross total resection (GTR) is not achieved in up to 50%, with resulting lower rates of progression-free survival (PFS).2
Surgery is the standard of care for meningiomas. Current postoperative management, especially in intermediate risk meningiomas, is currently not based on level 1 evidence. Patients with WHO grades 2 and 3 meningiomas are treated with a combination of surgery and postoperative irradiation.3 Treatment choice (radiotherapy [RT], re-operation, and active surveillance) is highly individualized and depends on tumor location, degree of progression, associated clinical symptoms, and the preference of the patient and treating physician.3,4 While re-operation can be an option in selected patients, overall patients undergoing re-operation for recurrent meningioma have been shown to have higher rates of postoperative complications and worse clinical outcomes.5,6 External beam radiation therapy (EBRT) and stereotactic radiosurgery (SRS) may improve clinical outcomes in patients with a higher risk of recurrence, including high-grade and recurring meningiomas.7 However, the role of EBRT for meningiomas is currently being evaluated.8 The NRG Oncology RTOG 0539 trial is an international Phase II trial assessing the benefits of RT in intermediate risk meningiomas, defined as recurrent WHO grade 1 meningiomas after either GTR or subtotal resection and new WHO grade 2 meningiomas after GTR. Rogers et al3 reported a 3-year PFS rate of 93.7% with minimal radiation-induced adverse events, validating the use of postoperative RT for intermediate-risk meningiomas. These results emphasize the importance of optimizing target delineation in postoperative RT planning of intermediate-risk meningiomas.
Accurate tumor delineation is critical for optimal radiation planning, as well as to minimize radiation-induced complications. Contrast-enhanced MR imaging is the standard of care for postoperative RT planning.3 However, MR imaging has many limitations regarding tumor delineation, particularly in the context of infiltrative or en plaque lesions, lesions with osseous or parenchymal invasion, and in differentiating residual tumor from postsurgical scarring or inflammation.7,9
Somatostatin receptor 2A (SSTR2A) is overexpressed in meningiomas and is a highly sensitive and specific meningioma biomarker.10,11 Gallium 68Ga-DOTATATE is a PET radiotracer binding SSTR2A with high affinity. It is widely used clinically for gastrointestinal neuroendocrine tumors, which also overexpress SSTR2A.12 68Ga-DOTATATE PET/CT demonstrated marked improvement in the sensitivity for detection of osseous involvement of meningiomas compared with MR imaging (98.5% versus 53.7%), while maintaining a high specificity (86.7% versus 93.3%).13 Most important, the improved sensitivity of 68Ga-DOTATATE PET for differentiating residual meningiomas from postsurgical dural thickening and enhancement may improve RT target volume delineation, thereby improving clinical outcomes. Improved tumor delineation reduces RT doses and RT burden to surrounding tissue and organs at risk by allowing more accurate targeting of tumors, thereby improving PFS while decreasing radiation-induced toxicity and tumor recurrence.7,14⇓⇓⇓-18
PET/MR imaging has been increasingly used clinically during the past decade. PET/MR imaging exposes patients to less radiation because MR imaging is used for attenuation correction obviating the need to acquire a CT scan. Additional key advantages include increased soft-tissue contrast and increased patient convenience and resource use, given that PET/MR imaging obviates the need for 2 separate studies.
We hypothesize that 68Ga-DOTATATE PET/MR imaging reduces health care costs due to a lower risk of both meningioma progression and radiation-induced complications after RT. To examine our hypothesis, we conducted a model-based cost-effectiveness analysis of 68Ga-DOTATATE PET/MR imaging for RT planning of intermediate-risk meningiomas in the US health care setting.
MATERIALS AND METHODS
Study Cohort
The modeled cohort represents patients with nonmalignant meningioma who are status post resection, with a mean age of 58 years based on the published mean age at diagnosis.15 The cohort was divided in our model into 2 broad groups based on treatment eligibility as recommended in the European Association of Neuro-Oncology (EANO): 1) RT-eligible patients: intermediate-risk meningioma defined in accordance to the RTOG 0539 ongoing Phase II clinical trial as patients with either newly diagnosed and completely resected (as assessed by MR imaging) WHO grade 2 meningiomas or recurrent WHO grade 1 meningiomas; and 2) patients under active surveillance: nonrecurrent WHO grade 1 meningioma.
WHO grade 3 meningiomas were not included in our study, given the lack of literature data specifically analyzing WHO grade 3 outcomes.
Decision-Analytic Model
We developed a decision-analytic model from a societal perspective in TreeAge Pro 2021 (TreeAge Software) to compare adjunct 68Ga-DOTATATE PET/MR imaging (“new strategy”) with MR imaging alone (“standard strategy”) for postresection meningioma evaluation and RT planning of intermediate-risk nonmalignant meningioma in terms of clinical outcomes and costs.
Treatment and management decisions followed the EANO guidelines on postresection RT for nonmalignant meningiomas.4 Patients in the model undergo tumor delineation and are selected for RT or active surveillance on the basis of the MR imaging or 68Ga-DOTATATE PET/MR imaging results as follows: 1) Patients with imaging findings showing the presence of residual intermediate-risk meningioma after subtotal resection are selected for RT with the EBRT or SRS technique;19 2) patients with imaging findings showing gross total resection are selected for active surveillance. Given the present lack of level 1 evidence for RT versus active surveillance in meningioma, we modeled the outcomes of these patients on the basis of the cohort in the study of Mirimanoff et al,20 reporting the recurrence and progression of patients with nonmalignant meningiomas following an operation.
The follow-up imaging used in both strategies follows the main recommendations endorsed by the National Comprehensive Cancer Network, consisting of imaging follow-up every 6 months for the first 2 years and every year thereafter.21
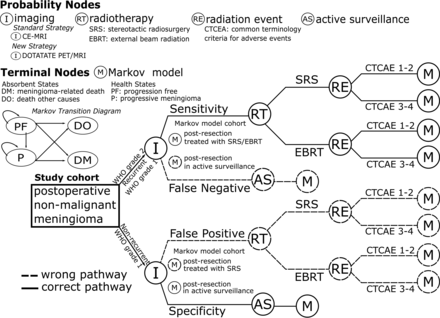
We incorporated the sensitivity and specificity of residual/recurrent meningioma detection following surgical resection for 68Ga-DOTATATE PET/MR imaging and MR imaging in the clinical pathways to assess the downstream effects of the treatment plan on the basis of the imaging results as illustrated in Fig 1.
Schematic representation of the decision-analytic model structure. The study cohort represents patients with nonmalignant meningioma status post resection. Patients with imaging findings of residual disease undergo RT. Following RT with either SRS or EBRT, the model accounts for the risk of toxicity as defined by the CTCAE classified as CTCAE 1–2 or CTCAE 3–4. The terminal nodes of the decision tree are Markov models with health states representing the status of the meningioma: progression-free, progression, or death (meningioma or other causes). The initial transition in the Markov models is determined by the assigned subcohort to a treatment plan (RT or active surveillance) based on the imaging results. Sensitivity and specificity were incorporated in the clinical pathways to properly assess the downstream effects of the imaging results on patients’ outcomes and costs, as illustrated in the diagram by the wrong and correct pathways. For example, patients with intermediate-risk nonmalignant meningiomas should be triaged to RT with either SRS or EBRT per the recommended guidelines (pathway represented by the solid-line branches). However, on the basis of the sensitivity of imaging (standard strategy: MR imaging; new strategy: 68Ga-DOTATATE PET-MR imaging), a proportion of these patients could be managed with active surveillance (pathway represented by the dashed-line branches), increasing their risk of progression in the Markov model. CE indicates contrast-enhanced.
Health Outcomes and Time Horizon
A Markov model was incorporated in the decision-analytic model to estimate the health outcomes and health care costs after intervention (RT or active surveillance). The health states of the Markov model were based on meningioma status and patient survival as follows: 1) stable (progression-free meningioma), 2) progression (worsened tumor burden), and 3) death (meningioma-related or other causes).
The Markov transitions were estimated with 1-year cycles. The initial transition corresponds to the observed health state after 1 year following intervention. Furthermore, the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE)22 was used to model radiation risks at 1 year following radiation therapy. Radiation risks were categorized as CTCAE 1–2 in severity and CTCAE 3–4 in severity, and values were based on reported data for SRS and EBRT in meningiomas.7,23⇓⇓-26
During the following 1-year cycles, patients could remain in the same health state (stable or progression), transition to a worse state (progression or meningioma-related death), or die (due to natural causes not associated with meningioma). The Markov transition probabilities were calculated with the PFS, overall survival, and mortality data of patients undergoing SRS or EBRT selected with MR imaging reported in the studies of Dohm et al26 and Pollock et al,25 respectively. Markov transitions for patients undergoing RT in the new strategy were based on the study by Zollner et al.23 Markov transitions for patients with intermediate-risk and nonrecurrent WHO 1 meningiomas following active surveillance were based on the studies of Dohm et al26 and Mirimanoff et al,20 respectively.
At every Markov transition, health outcomes were measured with utility weights representing the quality of life at each health state. The utilities for stable and progression states were based on a cost-effectiveness analysis of RT for high-risk, low-grade gliomas due to limited meningioma-specific published data.27 Costs included in the initial transition correspond to the cost of imaging, intervention and radiation-induced toxicity. Following the initial transition, the costs incorporated the cost of follow-up care (accounting for a visit to a physician and MR imaging every 6 months during the first 2 years and annually thereafter), progressive disease, and loss of productivity caused by premature meningioma-related death. Indirect costs were calculated with the retirement age at 72 years.28 A 3% discount rate was applied to both costs and utilities following the initial transition.29
The age-based mortality risk was obtained from the US Social Security Administration for the general population.30 Mortality hazard ratios were used in the model to adjust the mortality risk, given the presence of a meningioma.31 On the basis of data availability, a 10-year time horizon was implemented in the Markov model to assess the impact of the new strategy for treatment planning on the basis of the projected costs and outcomes.23,25,26,32
Input Parameters
The probabilities of experiencing a CTCAE 1–2 or 3–4 event for patients treated with SRS and EBRT in the new strategy were derived from the studies of Mahase et al7 and Zollner et al,23 respectively. Data from the studies of Dohm et al26 and Pollock et al25 were used in the standard strategy. The cost of 68Ga-DOTATATE was based on the tracer costs of neuroendocrine tumors established by the Centers for Medicare and Medicaid Services. The costs of PET/MR imaging, MR imaging, SRS, and EBRT were based on Current Procedural Terminology codes and the Centers for Medicare and Medicaid Services data. The input parameter values and sources can be found in the Online Supplemental Data. All costs are reported in US dollars.
Cost-Effectiveness and Sensitivity Analyses
The base case analysis was conducted for the modeled cohort with the model input values listed in the Online Supplemental Data. The quality-adjusted life-years (QALY) was selected to measure the effectiveness of the strategies,33 representing the projected quality of life, and it was estimated with the Markov model. Incremental cost-effectiveness ratio analyses were conducted to compare the strategies in which the society’s willingness to pay (WTP) for QALY gained were used to define cost-effectiveness. The US recommended WTP threshold values of $50,000/QALY and $100,000/QALY were implemented in our analyses.29,34
To evaluate the robustness of our results, we performed 1-way sensitivity and probabilistic sensitivity analyses to identify the key variables affecting the results in our model. The net monetary benefit metric was used in the 1-way sensitivity analyses.31 Probability distributions for the key model parameters were derived from conventional standards for the health utilities, probabilities, and costs. Furthermore, we evaluated the uncertainty of the results with respect to WTP thresholds ranging from $40,000/QALY to $400,000/QALY.35,36 The distribution type for the model input parameters is shown in the Online Supplemental Data.
RESULTS
Base Case Analyses
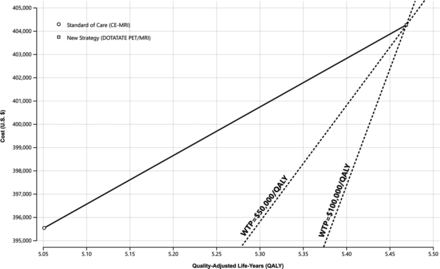
The cost-effectiveness results showed that the new strategy yields higher QALY (5.47 versus 5.05) at a higher cost ($404,260 versus $395,535) compared with the standard strategy, with an incremental cost-effectiveness ratio of $20,877 per QALY (Table). Consequently, the new strategy is cost-effective at a WTP of $50,000/QALY and $100,000/QALY (Fig 2).
Cost-effectiveness analysis summary for the base case
Cost-effectiveness analysis. The new strategy (68Ga-DOTATATE PET/MR imaging) yields higher QALY at a higher cost compared with the standard strategy (MR imaging). Furthermore, the new strategy is cost-effective at $50,000/QALY and $100,000/QALY. CE indicates contrast-enhanced.
Sensitivity Analyses
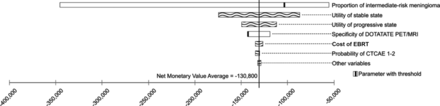
One-way sensitivity analyses indicated that the key variables contributing to variations in the net monetary values at both a WTP of $50,000/QALY (Fig 3) and $100,000/QALY are the proportion of intermediate-risk meningiomas in the modeled cohort, the utility for the stable and progressive health states, and the specificity of 68Ga-DOTATATE PET/MR imaging. Furthermore, the analyses identified that the new strategy is cost-effective at a WTP of $50,000/QALY ($100,000/QALY) when the proportion of intermediate-risk meningiomas of the modeled cohort is >12% (7%), the specificity of 68Ga-DOTATATE PET/MR imaging is >76% (58%), and the sensitivity of 68Ga-DOTATATE PET/MR imaging is >53% (44%); otherwise, the standard strategy is cost-effective.
Tornado analysis demonstrating the set of model parameters that play a role in the variation of the results measured with a net monetary benefit at a WTP of $50,000/QALY. Net monetary benefit = QALY × WTP – Cost.
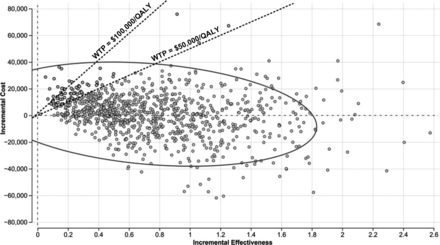
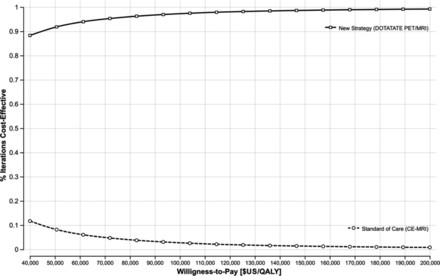
We conducted a probabilistic sensitivity analysis accounting for the collective variation of the input-model values with 100,000 iterations. The results (Fig 4) indicated that the new strategy is cost-effective at a WTP of $50,000/QALY ($100,000/QALY) with a probability of 91% (96%). Furthermore, the cost-effectiveness acceptability curve showed that the new strategy is cost-effective at WTP values ranging from $40,000/QALY to $200,000/QALY, with a probability of 88%–99%, respectively. These results highlight the robustness of the cost-effectiveness results (Fig 5).
Probabilistic sensitivity analysis considering the collective variation of costs, utilities, imaging parameters, and radiation-induced toxicity risk with 100,000 iterations. The probabilistic sensitivity analysis showed that the new strategy is cost-effective in 91% of the 100,000 iterations, demonstrating its cost-effectiveness at WTP = $50,000/QALY.
Cost-effectiveness acceptability curve with WTP values between $40,000/QALY and $200,000/QALY. The new strategy is consistently cost-effective across the WTP values used in our analysis. CE indicates contrast-enhanced.
DISCUSSION
Recent studies support adjunct 68Ga-DOTATATE PET use in RT planning for patients with intermediate-risk meningiomas.5,13⇓⇓-16 However, there is a dearth of literature exploring the cost-effectiveness of using 68Ga-DOTATATE for this purpose. Consequently, we analyzed the cost-effectiveness of 68Ga-DOTATATE PET/MR imaging to evaluate the practicality of incorporating it into clinical practice. Our study indicated that although the use of adjunct 68Ga-DOTATATE PET/MR imaging as part of the imaging work-up for postoperative RT planning in patients with intermediate-risk meningioma is associated with higher health care costs, its use is cost-effective from a societal perspective. More specifically, the difference in health benefits between the use of adjunct 68Ga-DOTATATE PET/MR imaging and MR imaging alone is 0.42 QALY, which translates to 5 additional months in perfect health.33,37 Moreover, the incremental cost-effectiveness ratio analysis indicated that 68Ga-DOTATATE PET/MR imaging is cost-effective at a WTP of >$20,877/QALY.
The sensitivity analyses established that the use of adjunct 68Ga-DOTATATE PET/MR imaging is cost-effective at a WTP of $50,000/QALY ($100,000/QALY) as long as the specificity of 68Ga-DOTATATE PET/MR imaging is >76% (58%) and the sensitivity of 68Ga-DOTATATE PET/MR imaging is >53% (44%). A recent diagnostic accuracy analysis of 68Ga-DOTATATE PET/MR imaging in 62 patients with meningiomas demonstrated a sensitivity and specificity of 86.7% and 80.5%, respectively, when using the standard uptake value ratio of the target lesion to superior sagittal sinus method.15 Therefore, the sensitivity and specificity in this published clinical cohort exceeded the thresholds needed to render PET/MR imaging cost-effective in our analysis. Most important, the probabilistic sensitivity analysis showed that 68Ga-DOTATATE PET/MR imaging is cost-effective with a probability of 91% (96%) at a WTP of $50,000/QALY ($100,000/QALY) when simultaneously varying the model input values according to their probability distribution.
Another insight from our analysis is that 68Ga-DOTATATE PET/MR imaging is cost-effective at a WTP of $50,000/QALY ($100,000/QALY) when the proportion of intermediate-risk meningiomas is >12% (7%). Population-based studies have shown that the proportion of WHO grade 2 meningiomas varies from 15% to 20%.38 Furthermore, recent studies have identified the increasing incidence trend of WHO grade 2 meningiomas, highlighting the importance of our results.39
Our model-based cost-effectiveness findings were similar to published results on 68Ga-DOTATATE PET/CT in patients with neuroendocrine tumors. A cost-effectiveness study performed by Froelich et al40 determined that 68Ga-DOTATATE PET/CT is cost-effective for detecting neuroendocrine tumors compared with Indium 111-pentetreotide SPECT/CT and CT alone, respectively. Froelich et al deemed it the most beneficial imaging approach for a diagnostic work-up of neuroendocrine tumors. The cost-consequence analysis conducted by Schreiter et al41 showed that the use of 68Ga-DOTATOC PET/CT for staging enteropancreatic neuroendocrine tumors reduces the overall use of health care resources.
Optimal radiosurgical planning relies on high-precision target volume delineation. In meningiomas, the gross target volume and clinical target volume are delineated on the basis of multimodal imaging. Although MR imaging is currently commonly used in clinical practice for tumor delineation, its accuracy is compromised in tumors located at high-contrast-enhancement areas or in tumors infiltrating the bone.13 Adjunct 68Ga-DOTATATE PET/CT and PET/MR imaging allow more accurate delineation of meningiomas,9,13,15⇓⇓-18 particularly in the postoperative setting, thereby improving gross target volume and clinical target volume delineation and reducing RT-associated toxicity. In a recently published pilot study, 68Ga-DOTATATE PET/MR imaging–based RT planning significantly reduced mean planning tumor volume to 11.12 cm3 from 71.39 cm3 on the basis of MR imaging alone.5 Moreover, the 68Ga-DOTATATE PET maximum standard uptake value correlated with the tumor growth rate in meningiomas, providing additional information related to tumor biology compared with MR imaging alone, probably of importance for the design of future therapeutic trials.42 Allocating resources to perform such trials is further supported if clinical validation leads to implementing a cost-effective management change that improves patient outcomes.
The main limitation of our model design is the lack of long-term data regarding the use of 68Ga-DOTATATE PET/MR imaging in patients with intermediate-risk meningiomas. Therefore, data extrapolations were required for modeling the Markov model transitions. Some of our input parameters were taken from studies using 68Ga-DOTATATE PET/CT rather than PET/MR imaging. Most important, prior studies regarding somatostatin analog PET radiotracers (such as 68Ga-DOTATATE, 68Ga-DOTA-TOC, 68Ga-DOTANOC) for other SSTR2A-positive neoplasms demonstrated that there is no significant difference in the detection rate between PET/CT and PET/MR imaging for a given radiotracer.43,44 In our model, we had to make assumptions such as considering that all patients with gross total resection were treated with active surveillance only. This may not always be the case in clinical practice because many radiation oncologists consider the use of RT following complete resection of WHO grade 2 meningiomas.3 Our model did not account for uncertain scenarios such as clinical situations with inconclusive imaging results as well as for meningioma location, which can affect clinical management and outcomes.24 Furthermore, there are extremely rare cases with MR imaging–positive, SSTR2A-negative findings and therefore 68Ga-DOTATATE PET–negative meningiomas.45 In clinical practice, information derived from 68Ga-DOTATATE PET/MR imaging has to be evaluated in conjunction with MR imaging findings and placed in the context of the individual patient. We assumed that radiation-associated toxicities would occur only in the first year postirradiation. Furthermore, we assumed that CTCAE 1–2 events would not transition to CTCAE 3–4 events due to data limitations. While delayed radiation toxicity can occur, such events tend to be very rare and it is unlikely that CTCAE 1–2 events will convert to CTCAE 3–4 events after 1 year of completion of radiation. The indirect costs were calculated assuming maximum retirement benefits, which occur at a retirement age of 72 years.46 Due to lack of data, we used standardized mortality ratios for meningiomas without the distinction of grade. Furthermore, for the surveillance cohort in our model, the Markov model was based on the study of Mirimanoff et al.20 While this study is older and not specifically limited to intermediate-risk meningiomas compared with later retrospective analyses such as that by Aghi et al,47 it has the largest sample size (225 patients) and has the needed data availability to model the transitions between health states (Fig 1). Given inclusion of all meningioma grades (except malignant meningiomas), the Mirimanoff et al cohort likely represents a closer approximation of the general clinical population of patients with meningiomas requiring postoperative management. Moreover, given that the limitations of MR imaging in the evaluation of meningiomas mostly pertain to the clinical context of postoperative recurrence and an invasive growth pattern,9 our model results are likely more conservative.
In this study, health care costs were based on a US setting. Thus, our findings may not be directly applied to other countries. However, our model can be adopted to other countries or regions by substituting region-specific parameters.
Significant advancements in molecular profiling of meningiomas have uncovered genetic and epigenetic biomarkers allowing more accurate prediction of tumor behavior compared with classic histopathologic approaches, including the mitotic rate and cytomorphologic criteria applied in the WHO classification scheme.48 Future studies may determine the need to incorporate molecular classifiers into decision-making regarding surveillance versus RT and may benefit from incorporating 68Ga-DOTATATE PET/MR imaging.49
Future studies, such as a cost-consequence analysis should be conducted for a comprehensive evaluation of the cost implications of 68Ga-DOTATATE PET/MR imaging in clinical practice. Future work should further incorporate molecular profiling data, which has recently demonstrated utility in predicting clinical outcomes in patients with meningiomas.50⇓⇓-53
CONCLUSIONS
Incorporating 68Ga-DOTATATE PET/MR imaging for meningioma management is cost-effective from a societal perspective and may improve RT planning, thereby improving clinical outcomes and reducing long-term costs due to RT-related complications.
Footnotes
J. Rodriguez and G. Martinez contributed equally to this work.
This work was partially supported by Novartis Pharmaceuticals, No.: CAAA501A0US05T, investigator-initiated trial grant; Principal Investigator: J. Ivanidze; Radiological Society of North America Resident Research Grant; Principal Investigator: M. Roytman; Radiological Society of North America Medical Student Research Grant; Principal Investigator: S. Kim; as well as the Clinical and Translational Science Center National Institutes of Health Predoctoral Training Award, Principal Investigator: A. Haghdel.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received November 8, 2022.
- Accepted after revision May 7, 2023.
- © 2023 by American Journal of Neuroradiology