Abstract
BACKGROUND AND PURPOSE: Neuromyelitis optica spectrum disorders are inflammatory demyelinating disorders with optic neuritis and/or longitudinally extensive transverse myelitis episodes. We now know that neuromyelitis optica spectrum disorders are associated with antibodies to aquaporin-4, which are highly concentrated on astrocytic end-feet at the blood-brain barrier. Immune-mediated disruption of the blood-brain barrier may manifest as contrast enhancement on brain MR imaging. We aimed to delineate the extent and frequency of contrast enhancement on brain MR imaging within 1 month of optic neuritis and/or longitudinally extensive transverse myelitis attacks and to correlate contrast enhancement with outcome measures.
MATERIALS AND METHODS: Brain MRIs of patients with neuromyelitis optica spectrum disorders were evaluated for patterns of contrast enhancement (periependymal, cloudlike, leptomeningeal, and so forth). The Fisher exact test was used to evaluate differences between the proportion of contrast enhancement in patients who were seropositive and seronegative for aquaporin-4 antibodies. The Mann-Whitney test was used to compare the annualized relapse rate and disease duration between patients with and without contrast enhancement and with and without seropositivity.
RESULTS: Brain MRIs of 77 patients were evaluated; 59 patients (10 males, 49 females) were scanned within 1 month of optic neuritis and/or longitudinally extensive transverse myelitis attacks and were included in the analysis. Forty-eight patients were seropositive, 9 were seronegative, and 2 were not tested for aquaporin-4 antibodies. Having brain contrast enhancement of any type during an acute attack was significantly associated with higher annualized relapse rates (P = .03) and marginally associated with shorter disease duration (P = .05). Having periependymal contrast enhancement was significantly associated with higher annualized relapse rates (P = .03).
CONCLUSIONS: Brain MRIs of patients with neuromyelitis optica spectrum disorders with contrast enhancement during an acute relapse of optic neuritis and/or longitudinally extensive transverse myelitis are associated with increased annual relapse rates.
ABBREVIATIONS:
- AQP4
- aquaporin-4
- ARR
- annualized relapse rate
- CE
- contrast enhancement
- IgG
- immunoglobulin G
- LETM
- longitudinally extensive transverse myelitis
- NMO
- neuromyelitis optica
- NMOSD
- NMO spectrum disorders
- ON
- optic neuritis
Neuromyelitis optica (NMO) is an inflammatory demyelinating disorder of the central nervous system,1 characterized by recurrent episodes of longitudinally extensive transverse myelitis (LETM) and/or optic neuritis (ON).2 Discovery of an NMO-specific autoantibody, NMO–immunoglobulin G (IgG), and its target autoantigen, aquaporin-4 (AQP4), have differentiated NMO from multiple sclerosis as a distinct disease entity.3 Moreover, given the high specificity of AQP4-IgG serology for clinically diagnosed NMO, such seropositivity was incorporated into the revised diagnostic criteria for NMO in 2006.1 The term “NMO spectrum disorders” (NMOSD) was introduced in 2007 to encompass broader phenotypes, including seropositive patients with coexisting autoimmune disorders and patients with limited or inaugural forms of NMO.4 The terms NMO and NMOSD were unified under a revised NMOSD definition in 2015.5 The unifying NMOSD diagnostic criteria allowed the diagnosis of NMOSD in patients without clinical involvement of the optic nerves or the spinal cord and stratified the diagnosis according to those with or without AQP4-IgG positivity.
While NMO was traditionally thought to be a disease exclusively involving the optic nerves and spinal cord, imaging abnormalities within the brain have been reported in a significant proportion of patients seropositive for AQP4-IgG, in regions with both high6,7 and low AQP4 expression.8 Lesions involving the diencephalon, area postrema, corpus callosum, hemispheric white matter, and corticospinal tracts have been reported.8 Specific patterns of contrast enhancement (CE) within the brain have also been reported in NMO, including pencil-thin,9 cloudlike,10 leptomeningeal,11 and perivascular enhancement (Figs 1 and 2).12 The current literature suggests a relatively low incidence of contrast-enhancing brain lesions in NMO.9⇓–11,13⇓⇓⇓⇓–18
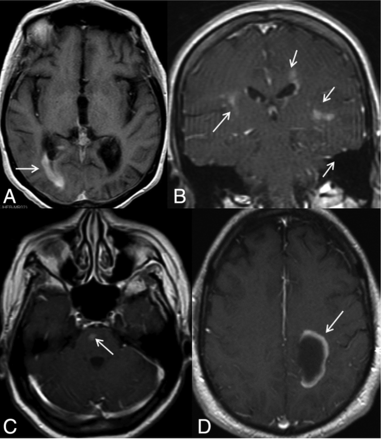
Different contrast-enhancement patterns are shown in this figure. A, A periependymal linear enhancement pattern can be seen surrounding the lateral, third or fourth ventricles, and/or cerebral aqueduct. Here we see right posterior periventricular enhancement (arrow). B, Periependymal enhancement and inhomogeneous, subtle parenchymal enhancement with ill-defined margins, so-called cloudlike enhancement (arrows). C, Isolated enhancement (arrow), D, Incomplete ring enhancement (arrow).
A 21-year-old male patient diagnosed with neuromyelitis optica. He initially presented with longitudinally extensive transverse myelitis when he was 12 years of age. MR imaging was performed at 14 years of age within 1 month of an acute LETM attack. Axial FLAIR image (A) shows a large region of increased signal abnormality within the pons, extending into the left middle cerebellar peduncle with expansion of the pons itself and the cerebellar hemisphere. Postcontrast T1-weighted image (B) shows cloudlike contrast enhancement (arrows).
However, in a large proportion of brain MRIs in those studies, whether they were acquired during an acute phase of the disease versus at any time point was not specified. Furthermore, the incidence of contrast-enhancing lesions in the brain during acute relapses of ON and/or LETM in patients with NMOSD has not been examined before, to our knowledge. Prior studies investigating predictors of relapse in patients with NMOSD have addressed factors that are either clinical or biochemical in nature, including AQP4-IgG seropositivity,19 female sex,20⇓–22 and older age of onset.23 In contrast, no MR imaging parameters have been shown to be associated with disease outcome.
In the current study, we aimed to delineate the extent and frequency of CE in the brain during acute attacks of ON and/or LETM. We also sought to determine whether detection of brain CE was associated with specific outcome measures, including disease duration and the annualized relapse rate (ARR).
Materials and Methods
Patients
A retrospective chart review was performed to identify patients with contrast-enhancing brain lesions between September 2001 and November 2013 at the Johns Hopkins NMO center. All patients identified were diagnosed with NMO or NMOSD based on the Wingerchuk et al 20061 or 20074 revised criteria, respectively.5 Institutional review board approval was obtained for the study. Electronic patient records were reviewed for demographic information, history of relapse, AQP4-IgG status, age at diagnosis, age at last follow-up, and the number of relapses.
Neuroimaging
MR imaging examinations were performed by using either 1.5T or 3T scanners (Philips Healthcare, Best, the Netherlands; GE Healthcare, Milwaukee, Wisconsin; and Siemens, Erlangen, Germany). T1WI, fast spin-echo T2WI, fast spin-echo FLAIR, and postgadolinium T1WIs were performed. A gadolinium contrast agent of 0.1 mL/kg was intravenously administered followed by a 20-mL saline injection. T1-weighted axial and coronal images were acquired without any delay after intravenous injection. The sagittal T1WIs were obtained with the following parameters: TR range = 520–696 ms, TE range = 4.6–14 ms, matrix size range = 192 × 192 to 512 × 196, FOV range = 190 × 190 mm to 240 × 240 mm, section thickness/spacing range = 1/1 to 5/7 mm. Axial T2WI was performed with the following parameters: TR range = 2500–7000 ms, TE range = 83–112 ms, matrix size range = 256 × 184 to 448 × 335, FOV range = 159 × 200 mm to 240 × 240 mm, section thickness/spacing range = 2/2 to 5/5 mm. A FLAIR sequence was obtained with the following parameters: TR = 6000 ms, TE = 120 ms, TI = 2000 ms, section thickness = 5 mm, FOV = 23 cm, matrix size = 256 × 256.
All brain MRIs were evaluated in consensus by 2 radiologists, a board-certified neuroradiologist (I.I.) and a radiologist (G.O.), with 10 and 4 years of experience, respectively. All patients had at least 1 brain MR imaging performed at our institution. Brain MRIs acquired within 1 month of the onset of the relapse were classified as imaging during an acute LETM and/or ON attack. CE was evaluated by using postgadolinium T1-weighted images. Brain CE was categorized in 6 specific patterns of enhancement: periependymal, cloudlike, leptomeningeal, isolated, ring, or other (Figs 1 and 2).
Statistical Analysis
The Fisher exact test was used to evaluate the difference between the proportions of patients with CE who were seropositive versus seronegative. A nonparametric Mann-Whitney test was used to compare the ARR and disease duration between those with and without CE. Regression analyses of the ARR with and without CE were also performed, with and without adjusting for age, sex, race, and AQP4-status. P values < .05 were considered statistically significant and were not adjusted for multiple analyses.
Results
Brain MRIs of 77 patients (11 males, 66 females) were evaluated for contrast enhancement. Fifty-nine patients (10 males, 49 females) underwent brain MR imaging within 1 month of the onset ON and/or LETM attack and were included in the final analysis. The mean age of patients was 47.8 years (range, 6–78 years). There were 35 African-American, 18 white, and 6 Hispanic (individuals from Mexico) individuals. Forty-eight patients were AQP4-IgG seropositive, 9 were seronegative, and the AQP4-IgG status was not checked in 2 of them. The ARR was not available for 1 patient.
Table 1 depicts the proportions of patients with CE in those with or without AQP4-IgG seropositivity during acute attacks. The Fisher exact test did not demonstrate significantly different proportions of CE in patients with or without AQP4-IgG seropositivity during acute attacks (P = .7). No significantly different proportions were noted when stratified by specific enhancement patterns (P = .7, data not shown).
Association between the proportion of patients with CE and the presence of AQP4-IgG seropositivitya
Tables 2 and 3 depict the association between the detection of CE during an acute phase and either disease duration or ARR. When imaged during the acute phase, patients demonstrating periependymal CE had significantly higher ARRs compared with those without periependymal CE (P = .03). Moreover, patients demonstrating any type of CE during the acute phase had significantly higher ARRs (P = .03) than those without.
Comparison of disease duration and ARR between patients with and without CE during an acute attack
Comparison of disease duration and ARR between patients with and without PCE during an acute attack
On the basis of the regression analyses, the unadjusted difference in ARRs between those with periependymal CE and those without it was 0.56 (95% CI, 0.07–1.05; P = .03). After we adjusted for age, sex, race, and AQP4 status, the difference was 0.60 (95% CI, 0.08–1.13; P = .03). The unadjusted difference in ARRs between those with any CE and without was 0.42 (95% CI, 0.04–0.80; P = .03). After we adjusted for age, sex, race, and AQP4 status, the difference was 0.41 (95% CI, 0.02–0.81; P = .04).
Table 4 shows the distribution of brain CE patterns among 59 patients who were scanned within 1 month of an ON and/or LETM attack. Brain CE was categorized and evaluated in 6 specific patterns of enhancement in the beginning of the study: periependymal, cloudlike, leptomeningeal, isolated, ring, or other (Figs 1 and 2). After excluding MRIs that were not obtained within 1 month of ON and/or LETM attack from the final analysis, we regrouped MRIs into 2 groups: a group with periependymal CE and a group with any type of CE. MRIs of 14 patients showed periependymal CE, and 21 patients showed any type of CE within 1 month of ON and/or LETM attacks.
Distribution of brain CE patterns among 59 patients with ON and/or LETM
Discussion
The current literature on NMO is limited in its description of neuroimaging features that may predict the outcome of disease.24 Most asymptomatic NMO brain lesions have not been shown to demonstrate enhancement, and the frequency of acute lesion-associated enhancement remains to be determined.23 This study demonstrates that approximately 63% of patients during an acute attack of ON and/or LETM may also show CE within the brain parenchyma. CE within the brain, when identified during an acute phase, is associated with a significantly increased ARR. The relapse rate during the first 2 years of the disease strongly determines the risk of an unfavorable outcome as defined by severe disability or death.25 Brain enhancement in patients during an acute ON and/or LETM may reflect a more severe underlying disease process compared with those without brain CE.
We found no significant difference in the propensity for CE in patients who were AQP4-IgG seropositive (64.6%) and seronegative (55.6%) (P = .7, Table 1). CE patterns of brain lesions in the current literature were described mostly in patients seropositive for AQP4-IgG and have been reported to range from 3% to 56%, excluding small case reports.3,7,9,10,13,16,17,26 Our study revealed a much higher proportion of CE, approximating 64.6% and 55.6% in patients seropositive and seronegative, respectively. A study investigating contrast-enhancing LETM lesions reported CE in 94% and 71% of seropositive and seronegative patients, respectively, though the authors did not specify the location of CE as within either the brain or spinal cord.27 To our knowledge, the current study is the largest cohort to report the frequency of contrast-enhancing brain lesions in both seropositive and seronegative patients during active ON and/or LETM relapse of NMO. The small sample of patients seronegative for AQP4-IgG in the current study may be contributing to lack of detection for a significant difference in the proportion of CE seen in seropositive and seronegative patients; however, that might be the case in prior studies failing to show a difference as well. Nevertheless, the number of patients seronegative for AQP4-IgG is always low compared with those who are seropositive; therefore, multi-institutional studies are needed to increase the sample size.
That AQP4 is highly expressed on astrocytic foot processes at the BBB and contributes to the maintenance of BBB integrity is well-described.3,28,29 Binding of AQP4-IgG to AQP4 in vitro has been shown to alter BBB permeability and astrocyte killing.30 Disruption of the BBB manifests as CE on brain MR imaging.31 Periependymal white matter is one of the most AQP4-rich regions of the brain; hence, the high prevalence of periependymal contrast enhancement in our cohort. Furthermore, AQP4-IgG is thought to be pathogenic only in proximity to CNS parenchyma, as evidenced by NMO-like histopathology in animal models in those that received direct administration of AQP4-IgG into the CNS. In contrast, peripheral administration had no effect.32 The presence and levels of AQP4-IgG in CSF are associated and correlated with those in serum during acute relapses.33,34 For example, AQP4-IgG is detectable in the CSF of most seropositive patients with serum titers of >1:250 during an acute relapse.35 Moreover, the amount of CSF AQP4-IgG is correlated with astrocyte damage and BBB breakdown.34 Therefore, it is possible that those with lower serum titers or those not in acute relapse in the current study may not have detectable or significant CSF levels of AQP4-IgG to lead to the BBB disruption and consequent CE. Most interesting, there have been reports of patients with NMOSD who are AQP4-IgG positive in the serum for many years before the onset of symptomatic disease.36 The poor correlation between the presence and level of serum and CSF titers of AQP4-IgG may be contributing to the lack of significantly different proportions of CE in seropositive and seronegative patients in our study. It may also be contributing to the high interstudy variability in the reports of the percentage of CE observed in patients with NMOSD.3,7,9,10,13,16,17,26,27
Most important, the association between CE during the acute phase and ARR may be confounded because those who underwent brain MRIs during an acute phase may have warranted more immediate imaging because they may have been inherently sicker. Findings may be further confounded by other clinical characteristics. For example, longer intervals between the first and second attack,37 older age at onset,23 patients of African origin,38 female sex,20⇓–22 and AQP4-IgG seropositivity19 are associated with worse outcomes and/or higher relapse rates. However, other studies failed to find AQP4-IgG status as a predictor of outcome. Jarius et al39 found that AQP4-IgG status did not differ significantly with regard to time to relapse or ARR. Jiao et al40 found that the effect of seropositive status on the relapse rate and disability outcome did not differ. Responses to plasmapheresis based on AQP4-IgG were also not significantly different.41 Regardless of the discrepant prognostic findings of AQP4-IgG in the existing literature, in our study, a periependymal pattern of CE and the presence of any pattern of CE in the acute phase remained significant predictors of higher ARRs after adjusting for AQP4 status, as well as age, sex, and race, on multivariable analysis.
Enhancement patterns of brain lesions in NMO have some unique features and sometimes, in the presence of characteristic T2 lesions, might aid in making a specific diagnosis. Patchy CE with blurred margins, so-called “cloudlike enhancement,” is the most commonly reported enhancement pattern in the literature.10 More recently, linear periependymal CE, so called “pencil-thin enhancement,” and leptomeningeal CE were proposed as more specific patterns than cloudlike enhancement.9,11 Isolated CE and ring and open-ring CE are considered specific to MS, and they are rarely seen in patients with NMOSD. However, although rare, these intense, well-defined CE patterns have been described before, especially in seronegative patients with NMOSD.18
The main limitation of our study is the retrospective design, and factors that have been described in the literature associated with outcomes such as seropositivity status, sex, race, and age at onset may be potential confounders and were not accounted for. There is a possible selection bias based on a group of patients with NMOSD who required brain MR imaging, which may reflect a different subpopulation than that not requiring brain MRIs. The threshold of 1 month as the criterion for an acute attack may be arbitrary, given the lack of information in records available to more accurately assess the patients' clinical statuses and may thus misrepresent these statuses in the current study. Furthermore, the current study was originally conducted before the introduction of the more inclusive revised diagnostic criteria for NMOSD of 2015.5 Rather, included patients were based on the 2006 diagnostic criteria; therefore, the current study does not account for patients who may now qualify as diagnostic for NMOSD under the 2015 criteria.
Conclusions
Detection of CE in postgadolinium T1-weighted brain imaging within 1 month of onset of an acute ON and/or LETM is associated with higher ARRs. CE is an important marker reflecting the underlying pathogenic process of NMOSD. Although no significant association was found between CE and AQP4-IgG serostatus, the strong interplay among the BBB disruption, AQP4-IgG deposition, and CE warrants further investigation with a larger multicenter cohort to determine the prognostic role that CE may play as a predictor of outcome and its correlation with clinical severity.
Footnotes
Disclosures: Carol B. Thompson—RELATED: Grant: National Institutes of Health-Clinical and Translational Science Awards 1UL1TR001079 to Johns Hopkins, Comments: The grant provides limited free statistical consultation to researchers.* Maureen Mealy—UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Consortium of Multiple Sclerosis Centers, Comments: honoraria. Michael Levy—UNRELATED: Grant: National Institutes of Health, Genzyme/Sanofi, Alnylam Pharmaceuticals, Alexion Pharmaceuticals, MedImmune, Shire*; Consulting Fee or Honorarium: Genzyme/Sanofi, Alexion Pharmaceuticals; Fees for Participation in Review Activities such as Data Monitoring Boards, Statistical Analysis, Endpoint Committees, and the Like: Quest Diagnostics; UNRELATED: Board Membership: Acorda Therapeutic, Alexion Pharmaceuticals, Chugai Pharmaceuticals, Asterias Biotherapeutics; Payment for Development of Educational Presentations: Quest Diagnostics. Izlem Izbudak—UNRELATED: Consultancy: Alexion Pharmaceuticals, Comments: consulting neuroradiologist for the NMO 301 and NMO 302 PREVENT trial sponsored by Alexion Pharmaceuticals; Grants/Grants Pending: Siemens, Comments: DTI of the spinal cord in compressive myelopathy, Principal Investigator of the grant*; Other: Biogen, Comments: neuroradiologist of MS PATHS study partner institute.* *Money paid to the institution.
Carol Thompson received a National Institutes of Health–Clinical and Translational Science Awards grant 1UL1TR001079 (to Johns Hopkins). Maureen Mealy received honoraria from the International Organization of Multiple Sclerosis Nurses and EMD Serono. Michael Levy received research support from the Guthy-Jackson Charitable Foundation, ViroPharma, Acorda Therapeutics, Sanofi, Neuralstem, and Genentech and serves as a consultant for Chugai Pharmaceuticals, GlaxoSmithKline, Alexion Pharmaceuticals, and MedImmune.
Paper previously presented in part as an oral presentation at: Annual Meeting of the American Society of Neuroradiology and the Foundation of the ASNR Symposium, April 25–30, 2015, Chicago, Illinois. The presentation number was O-444.
Indicates open access to non-subscribers at www.ajnr.org
REFERENCES
- Received December 22, 2016.
- Accepted after revision January 14, 2017.
- © 2017 by American Journal of Neuroradiology